Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A material (which is a lot like lead) melts at 327 C. Its latent heat of fusion is 6 cal g, its density is
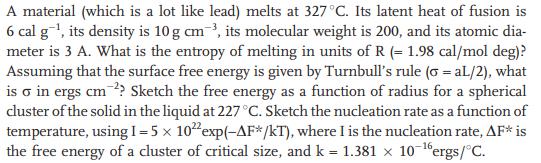
A material (which is a lot like lead) melts at 327 C. Its latent heat of fusion is 6 cal g, its density is 10 g cm, its molecular weight is 200, and its atomic dia- meter is 3 A. What is the entropy of melting in units of R (= 1.98 cal/mol deg)? Assuming that the surface free energy is given by Turnbull's rule (o = aL/2), what is o in ergs cm2? Sketch the free energy as a function of radius for a spherical cluster of the solid in the liquid at 227 C. Sketch the nucleation rate as a function of temperature, using 1 = 5 x 1022 exp(-AF*/kT), where I is the nucleation rate, AF* is the free energy of a cluster of critical size, and k = 1.381 x 10-6 ergs/C.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the entropy of melting we can use the formula S H T where S is the entropy of melting H is the enthalpy of fusion and T is the temperature at ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


