Answered step by step
Verified Expert Solution
Question
1 Approved Answer
do all problems PES Allowed Attempts 5 Question 16 When a solution of potassium hydroxide and zinc chloride are mixed what precipitate if any is
do all problems 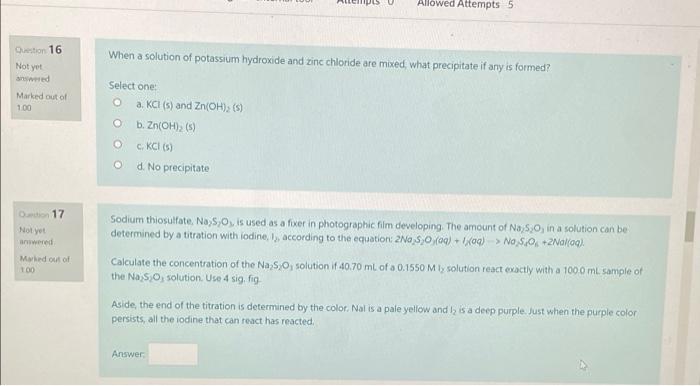
PES Allowed Attempts 5 Question 16 When a solution of potassium hydroxide and zinc chloride are mixed what precipitate if any is formed? Not yet wed Marked out of 100 Select one @ a. KCI (s) and Zn(OH), (s) @ b. Zn(OH)2 (5) cKCI) O d. No precipitate Do 17 Not awwered Med out of 100 Sodium thiosulfate, Na,s,, is used as a fixer in photographic film developing. The amount of Na, 530, in a solution can be determined by a titration with lodine, I, according to the equation: 2N0 5,0 (09) +1:00) > Na $+2Nal(aq). Calculate the concentration of the Na S, solution f 40.70 mL of a 0.1550 Mly solution react exactly with a 1000 m. sample of the Na: 5,0, solution. Use 4 sig. fig Aside, the end of the titration is determined by the color. Nalis a pale yellow and I, a deep purple. Just when the purple color persists, all the iodine that can react has reacted 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


