Answered step by step
Verified Expert Solution
Question
1 Approved Answer
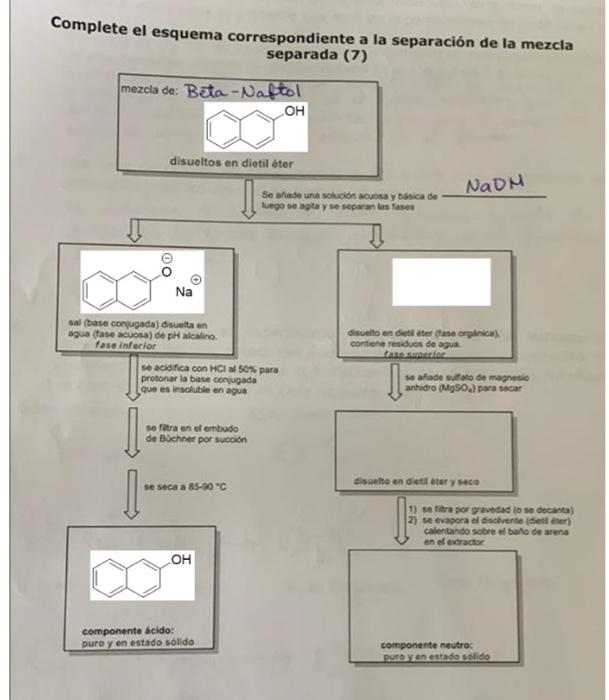
DO COLUMN BOXES ON THE RIGHT An aqueous and basic NaOH solution is added to it, then it is stirred and the phases are separated.
DO COLUMN BOXES ON THE RIGHT
An aqueous and basic NaOH solution is added to it, then it is stirred and the phases are separated.
Box row from left:
Salt (conjugate base) dissolved in water (aqueous phase) of alkaline pH (lower phase) IS acidified with 50% HCL to protonate the conjugate base which is insoluble in water. It is filtered in the Buchner funnel by suction and dried at 85-90 C.
box 2 from left
Pure and solid acid component.
Box column right
dissolved in diethyl ether (organic phase) contains residual water
upper phase
dissolved in diethyl ether and dried
1) filtered by gravity
2) the solvent (diethyl ether) is evaporated by heating over the sand bath in the extractor.
Neutral component:
pure and solid
translation: Draw and Write the two ACID-BASE EQUATIONS OF THE PROCEDURE OF EXTRACTION OF THE ALLOCATED MIXTURE. Show the electronic flow. An aqueous and basic NaOH solution is added to it, then it is stirred and the phases are separated.
Complete el esquema correspondiente a la separacin de la mezcla separada (7) mezcla de: Beta - Naftal OH disueltos en dietilter NADH Se alade una solucin acuosa y basica de luego se agta y se separan les fases 00 Na sal (base conjugada) disuelta en agua (fase acuosa) de pH alcalino fase inferior se acidifica con HCl al 50% para protonar la base conjugada que es insoluble en agua discelto en dietter (fase orginical contiene residuos de agua fase canade state demagnesis anhidro (MgSO) para secar so fitra en el embudo de Bchner por succion disuelto en distillater y seco se seca a 85-90 C 1) sera por gravedad (o se decanta) se evapora el disolverteiler) calentando sobre el ballo de arena en el extractor OH componente cido: puro y en estado solido componente neutro: puro y en estado solido Box row from left:
Salt (conjugate base) dissolved in water (aqueous phase) of alkaline pH (lower phase) IS acidified with 50% HCL to protonate the conjugate base which is insoluble in water. It is filtered in the Buchner funnel by suction and dried at 85-90 C.
box 2 from left
Pure and solid acid component.
Box column right
dissolved in diethyl ether (organic phase) contains residual water
upper phase
dissolved in diethyl ether and dried
1) filtered by gravity
2) the solvent (diethyl ether) is evaporated by heating over the sand bath in the extractor.
Neutral component:
pure and solid
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


