Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Do not copy answers from other people! Diederichsen and Hatton (2022) analyze the steady-state operation of this tubular membrane, into the interior of which is
Do not copy answers from other people!
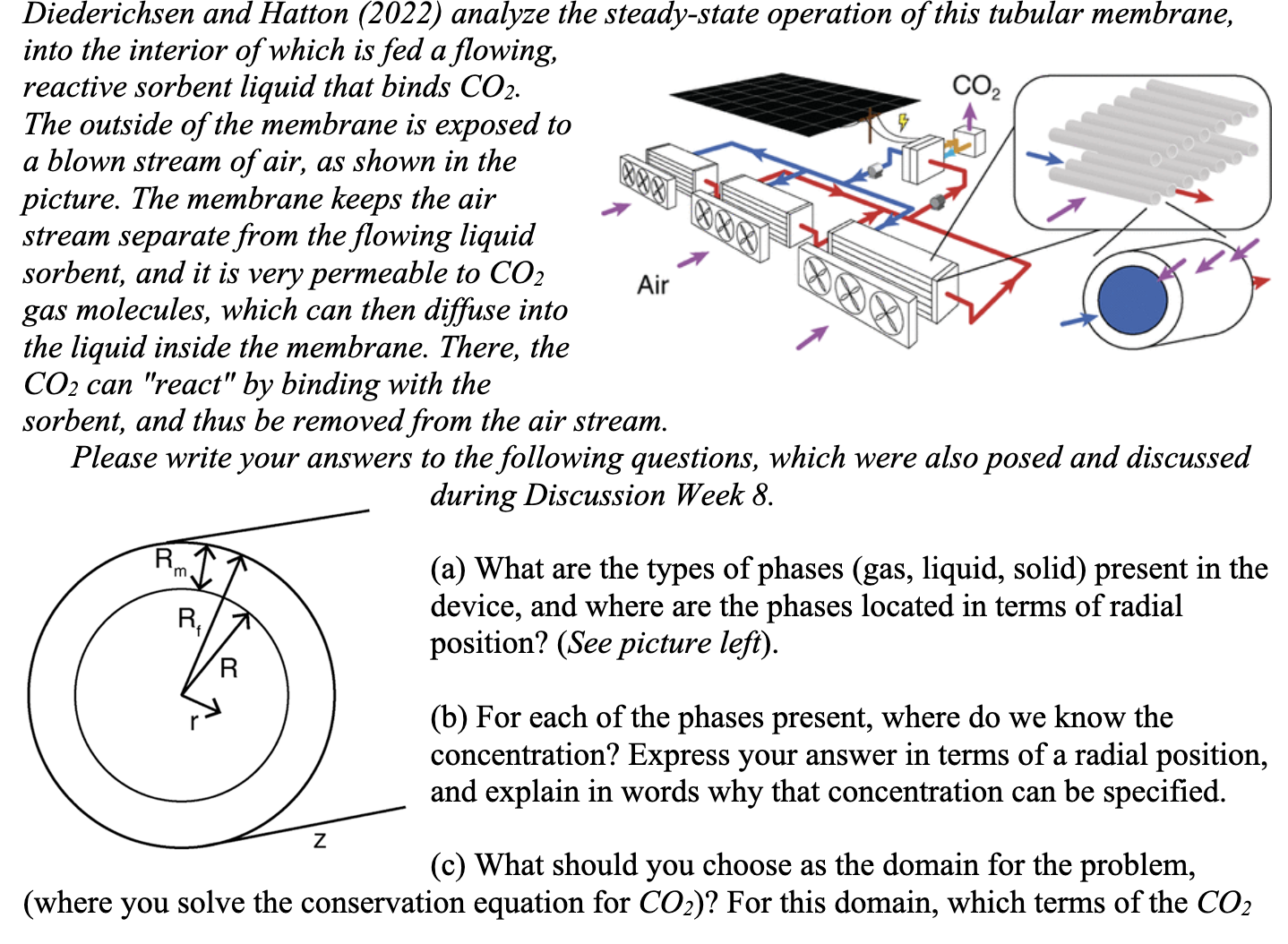
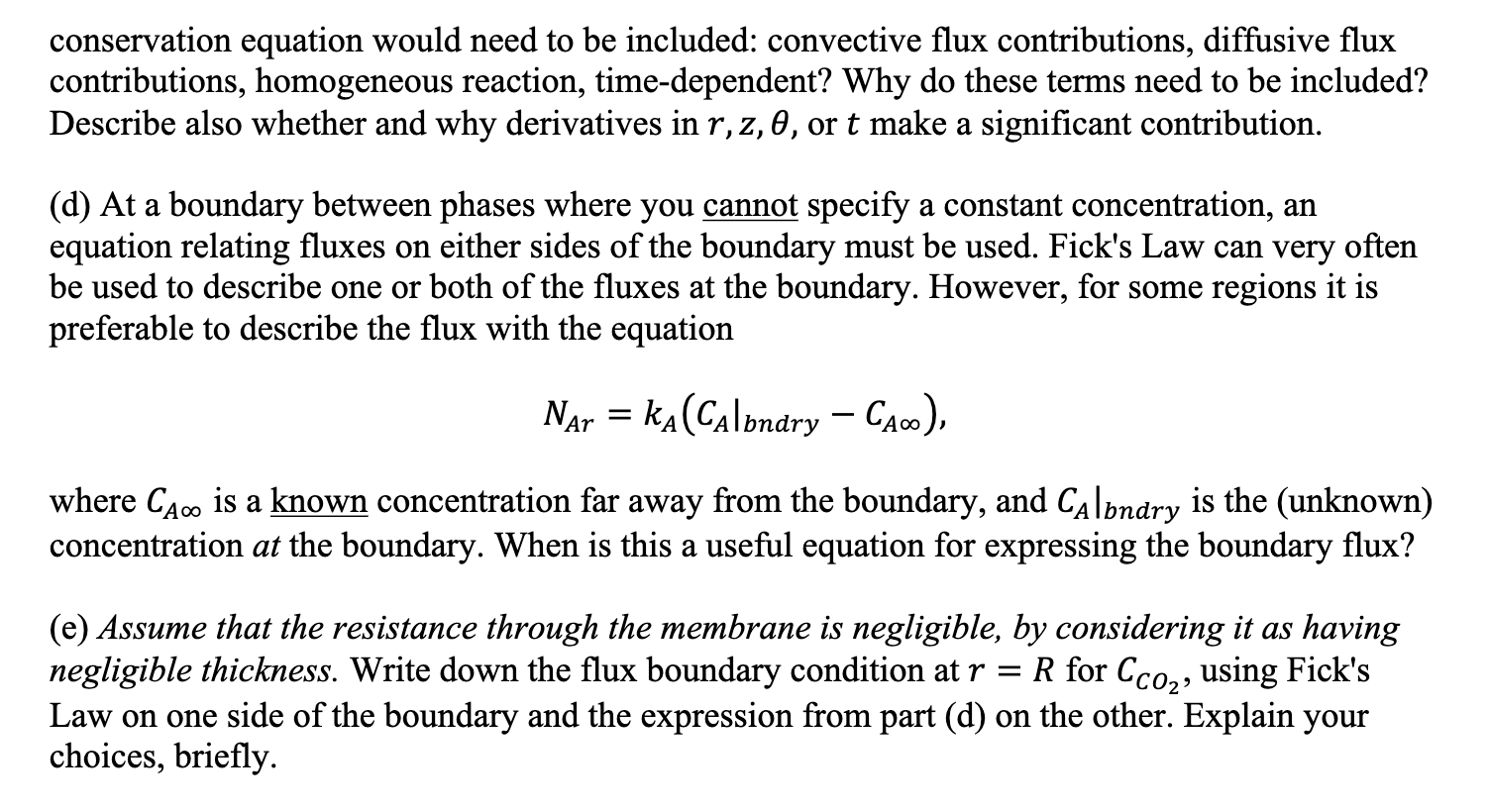 Diederichsen and Hatton (2022) analyze the steady-state operation of this tubular membrane, into the interior of which is fed a flowing, reactive sorbent liquid that binds CO2. The outside of the membrane is exposed to a blown stream of air, as shown in the picture. The membrane keeps the air stream separate from the flowing liquid sorbent, and it is very permeable to CO2 gas molecules, which can then diffuse into the liquid inside the membrane. There, the CO2 can "react" by binding with the sorbent, and thus be removed from the air stream. Please write your answers to the following questions, which were also posed and discussed during Discussion Week 8 . (a) What are the types of phases (gas, liquid, solid) present in the device, and where are the phases located in terms of radial position? (See picture left). (b) For each of the phases present, where do we know the concentration? Express your answer in terms of a radial position, and explain in words why that concentration can be specified. (c) What should you choose as the domain for the problem, (where you solve the conservation equation for CO2 )? For this domain, which terms of the CO2 conservation equation would need to be included: convective flux contributions, diffusive flux contributions, homogeneous reaction, time-dependent? Why do these terms need to be included? Describe also whether and why derivatives in r,z,, or t make a significant contribution. (d) At a boundary between phases where you cannot specify a constant concentration, an equation relating fluxes on either sides of the boundary must be used. Fick's Law can very often be used to describe one or both of the fluxes at the boundary. However, for some regions it is preferable to describe the flux with the equation NAr=kA(CAbndryCA) where CA is a known concentration far away from the boundary, and CAbndry is the (unknown) concentration at the boundary. When is this a useful equation for expressing the boundary flux? (e) Assume that the resistance through the membrane is negligible, by considering it as having negligible thickness. Write down the flux boundary condition at r=R for CCO2, using Fick's Law on one side of the boundary and the expression from part (d) on the other. Explain your choices, briefly
Diederichsen and Hatton (2022) analyze the steady-state operation of this tubular membrane, into the interior of which is fed a flowing, reactive sorbent liquid that binds CO2. The outside of the membrane is exposed to a blown stream of air, as shown in the picture. The membrane keeps the air stream separate from the flowing liquid sorbent, and it is very permeable to CO2 gas molecules, which can then diffuse into the liquid inside the membrane. There, the CO2 can "react" by binding with the sorbent, and thus be removed from the air stream. Please write your answers to the following questions, which were also posed and discussed during Discussion Week 8 . (a) What are the types of phases (gas, liquid, solid) present in the device, and where are the phases located in terms of radial position? (See picture left). (b) For each of the phases present, where do we know the concentration? Express your answer in terms of a radial position, and explain in words why that concentration can be specified. (c) What should you choose as the domain for the problem, (where you solve the conservation equation for CO2 )? For this domain, which terms of the CO2 conservation equation would need to be included: convective flux contributions, diffusive flux contributions, homogeneous reaction, time-dependent? Why do these terms need to be included? Describe also whether and why derivatives in r,z,, or t make a significant contribution. (d) At a boundary between phases where you cannot specify a constant concentration, an equation relating fluxes on either sides of the boundary must be used. Fick's Law can very often be used to describe one or both of the fluxes at the boundary. However, for some regions it is preferable to describe the flux with the equation NAr=kA(CAbndryCA) where CA is a known concentration far away from the boundary, and CAbndry is the (unknown) concentration at the boundary. When is this a useful equation for expressing the boundary flux? (e) Assume that the resistance through the membrane is negligible, by considering it as having negligible thickness. Write down the flux boundary condition at r=R for CCO2, using Fick's Law on one side of the boundary and the expression from part (d) on the other. Explain your choices, briefly Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


