Answered step by step
Verified Expert Solution
Question
1 Approved Answer
do the deafut table URGENT PLEASE Instructions: Solve the following problem, showing all the steps you made in the procedure. Be careful with units. Give
do the "deafut" table 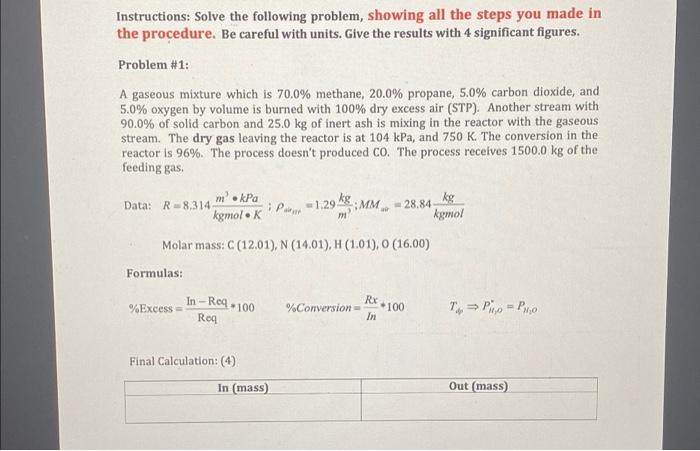









URGENT PLEASE
Instructions: Solve the following problem, showing all the steps you made in the procedure. Be careful with units. Give the results with 4 significant figures. Problem #1: A gaseous mixture which is 70.0% methane, 20.0% propane, 5.0% carbon dioxide, and 5.0% oxygen by volume is burned with 100% dry excess air (STP). Another stream with 90.0% of solid carbon and 25.0 kg of inert ash is mixing in the reactor with the gaseous stream. The dry gas leaving the reactor is at 104 kPa, and 750 K. The conversion in the reactor is 96%. The process doesn't produced Co. The process receives 1500.0 kg of the feeding gas. Data: R-8,314 m'kPa kgmolek P-1.29 kg; MM, - 28.84 kg komol Molar mass: C (12.01), N (14.01), H (1.01), (16.00) Formulas: In --Req-1 100 Rx %Excess = %Conversion 100 To Pio - Pho Roq Final Calculation: 4) In (mass) Out (mass) Answer the following questions: 1. Sketch the diagram of the process. (6) Process 2. Give a basis (complete) for the process. (3) 3. Give the degree of freedom analysis. (5) 4. Write and balance the three reactions in the process. (6) Determine all molar and weight compositions for each stream, using material balances. (Be careful with all conversion specifications). (11) Give a table with the information of all streams ("default"): (10) Calculate the following: 1. Calculate the molar selectivity of CO2 relative to H20. (2) 2. Determine the average molecular weight of the dry gas leaving the process. (2) 3. Give the molar composition of the gas leaving the process, assuming as a stack (wet) gas. (5) 4. Determine the average molecular weight of the stack gas leaving the process. (2) 5. Determine the specific volume of the stack gas (m /kg). (4) 6. Determine the specific gravity of the stack gas. The air reference density is 1.29 kg/m3. (3) 7. Determine the partial pressure of water in the stack gas (kPa). (2) 8. Determine the dew point temperature of the stack gas (K) (5) 9. Determine the conversion percent of Oz Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


