Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Don't even know where to start step wise Section D: Density as a Conversion factor For the following problems, you must use dimensional analysis to
Don't even know where to start step wise 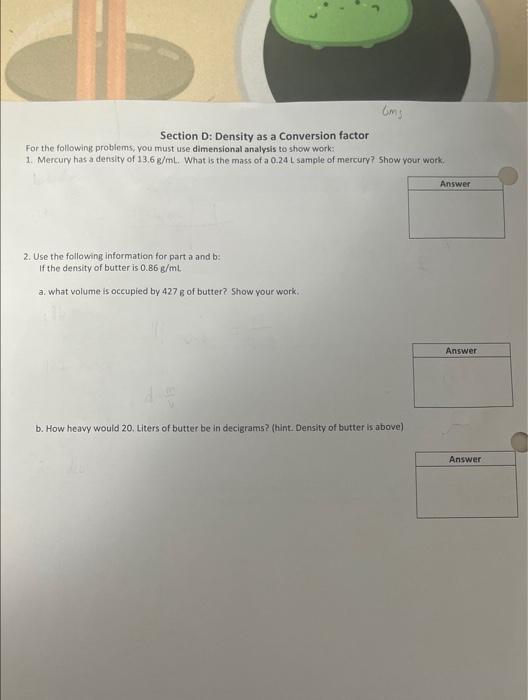
Section D: Density as a Conversion factor For the following problems, you must use dimensional analysis to show work: 1. Mercury has a density of 13.6g/mL. What is the mass of a 0.24L sample of mercury? Show your work. 2. Use the following information for part a and b : If the density of butter is 0.86g/mL a. what volume is occupied by 427g of butter? Show your work. b. How heavy would 20, Liters of butter be in decigrams? (hint. Density of butter is above) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


