Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Don't need graphs, please show work on problems. Procedure You will perform four separate trials, each involving a different solution. PREPARE AND WORK WITH ONE

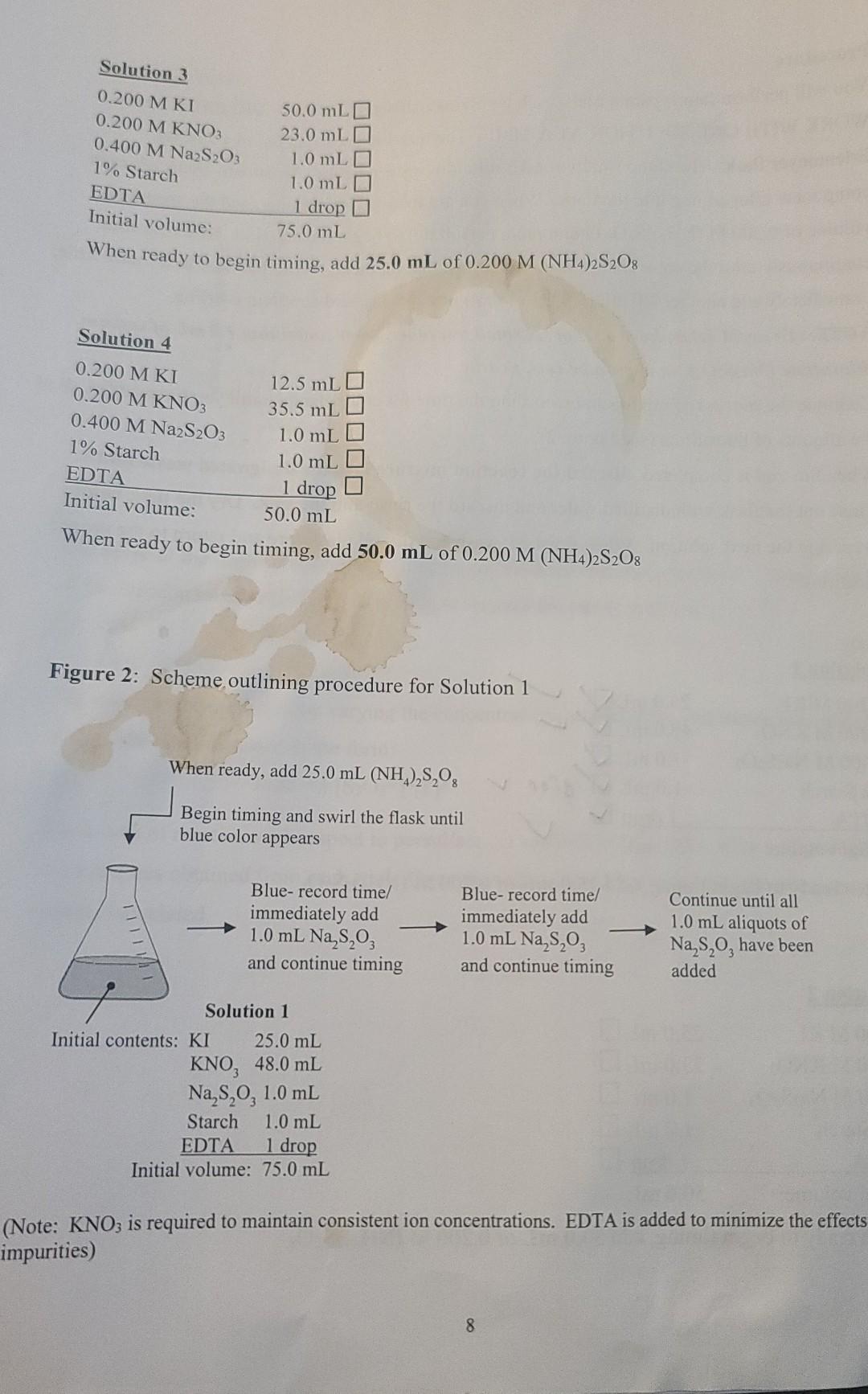


Don't need graphs, please show work on problems.


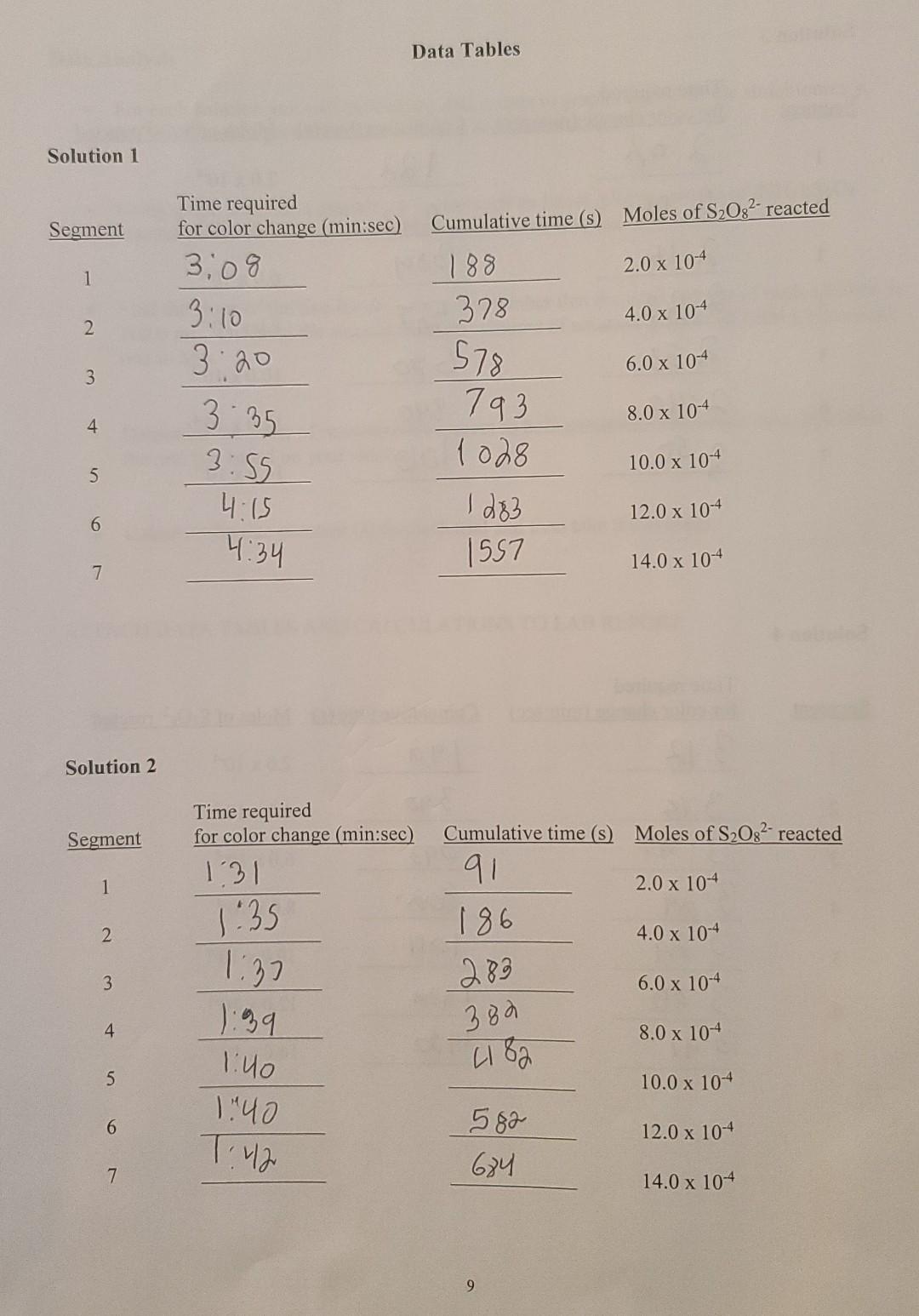
Procedure You will perform four separate trials, each involving a different solution. PREPARE AND WORK WITH ONE SOLUTION AT A TIME. The reaction is carried out in a 250 mL Erlenmeyer flask. The composition of each solution is given below. Be sure to check off each component after adding it to the flask. When you are ready to start timing, add the specified volume of 0.200 M (NH4)2S2O: (ammonium persulfate) to begin the reaction. Swirl the flask continuously until the color change is observed. At that moment, record the time in the data table, immediately add another 1.0 mL (4.0 x 104 mol) of Na2S2O3, and continue swirling. NOTE: To avoid delay, have a set of six small test tubes, each containing 1.0 mL of sodium thiosulfate (Na2S2O3), in a test tube rack nearby. Continue the process of mixing and recording the time for color change until you have added all of the aliquots of thiosulfate (see Figure 2). When the trial is completed, discard the reaction mixture into the designated waste bottle. Rinse out the flask with distilled water and discard the rinse into the sink. Dry the flask before preparing the next solution. When finished, wash and dry all glassware and return to the proper locations. Solution 1 25.0 mL 0.200 MKI 0.200 M KNO3 0.400 M Na2S2O3 1% Starch EDTA Initial volume: 48.0 mL V 1.0 mL 1.0 mL o drop r 1 drop 75.0 mL When ready to begin timing, add 25.0 mL of 0.200 M (NH4)2S208 Solution 2 0.200 M KI 25.0 mL 0.200 M KNO3 23.0 mL o 0.400 M Na2S2O3 1.0 mL O 1% Starch 1.0 mL EDTA 1 drop O Initial volume: 50.0 mL When ready to begin timing, add 50.0 mL of 0.200 M (NH4)2S208 Solution 3 0.200 MKI 0.200 M KNO3 0.400 M Na2S2O3 1% Starch EDTA Initial volume: 50.0 mLO 23.0 mL 1.0 mLO 1.0 mL 1 drop 75.0 mL When ready to begin timing, add 25.0 mL of 0.200 M (NH4)2S2O: Solution 4 12.5 mL 35.5 mL 1.0 mL 1.0 mL 1 drop 50.0 mL When ready to begin timing, add 50.0 mL of 0.200 M (NH4)2S2O: 0.200 MKI 0.200 M KNO3 0.400 M Na2S2O3 1% Starch EDTA Initial volume: Figure 2: Scheme outlining procedure for Solution 1 When ready, add 25.0 mL (NH2,8,0, Begin timing and swirl the flask until blue color appears Blue-record time/ immediately add 1.0 mL Na,$,03 and continue timing Blue- record time/ immediately add 1.0 mL Na S,03 and continue timing Continue until all 1.0 mL aliquots of Na,s,o, have been added Solution 1 Initial contents: KI 25.0 mL KNO, 48.0 mL Na,3,0, 1.0 mL Starch 1.0 mL EDTA 1 drop Initial volume: 75.0 mL (Note: KNO3 is required to maintain consistent ion concentrations. EDTA is added to minimize the effects impurities) 8 Data Tables Solution 1 Segment 1 Time required for color change (min:sec) 3,08 3:10 3:20 Cumulative time (s) Moles of S2O32- reacted 188 2.0 x 10-4 378 4.0 x 10-4 2 6.0 x 10-4 3 4 3.35 3.55 4:15 578 793 1028 I d&B 557 8.0 x 10-4 10.0 x 104 12.0 x 10-4 5 6 4:34 14.0 x 104 7 Solution 2 Segment Time required for color change (min:sec) 1:31 1 1:35 Cumulative time (s) Moles of S2O32-reacted 91 2.0 x 104 186 4.0 x 104 6.0 x 10-4 2 1:37 3 283 380 2182 4 8.0 x 104 5 1:40 1 " 10.0 x 10+ 6 582 684 12.0 x 10-4 T:42 7 14.0 x 104 9 Data Tables Solution 1 Segment Cumulative time (S) Moles of S2O32- reacted Time required for color change (min:sec) 3,08 2.0 x 104 1 188 378 4.0 x 10-4 2 3:10 3:20 6.0 x 104 3 578 793 8.0 x 10-4 4 3-35 3:55 10.0 x 104 5 4.15 1028 I do 1557 12.0 x 10-4 6 4:34 14.0 x 10+ 7 Solution 2 Segment Time required for color change (min:sec) 131 1 1.35 2. 3 Cumulative time (s) Moles of S2O32- reacted 91 2.0x 10+ 186 4.0 x 104 289 6.0 x 104 380 8.0 x 104 2182 10.0 x 104 582 12.0 x 10-4 684 14.0 x 10-4 1:32 ):39 1:40 140 4 5 6 T: 42 Data Analysis . For each solution you will have seven data points to graph. Based on the stoichiometry, 2.0 x 10mol of persulfate has reacted at each interval. Using graph paper or a graphing program such as Excel, plot the moles of (NH4)2S2O: reacted versus cumulative time in seconds for each solution. Find the slope of the line for each trial. Remember that the total volume of each solution is 100.0 mL. Dividing the slope by the total volume of solution in liters (0.1000 L) gives the rate in M/s. Determine the order of reaction with respect to both persulfate and iodide ions, and write the rate law based on your results. e Calculate the rate constant (k) for each trial and then take the average. 1. Enter the rate of reaction determined for each solution. Solution 1: M/s Solution 2: M/s Solution 3: M/s Solution 4: M's 2. Compare the reaction rates between solutions 1 and 2. Note that persulfate concentration was doubled in solution 2, while iodide concentration was held constant. 3. Compare the reaction rates between solutions 1 and 3. Note that iodide concentration was doubled in solution 3, while persulfate concentration was held constant. 4. Compare the reaction rates between solutions 2 and 4. Note that iodide concentration was cut in half in solution 4, while persulfate concentration was held constant. 5. Write the experimental rate law with respect to persulfate and iodide. Solution 3 Segment Time required for color change (min sec) 2.02 Cumulative time (s) Moles of S2O32 reacted 122. 2.0 x 10-4 1 2 2:06 4.0 x 104 2.48 384 3 2:16 6.0 x 10-4 4. 527 8.0 x 10-4 5 2:23 2:33 2:40 680 10.0 x 10-4 6 12.0 x 104 840 lola 7 2.50 14.0 x 10-4 Solution 4 Segment Time required for color change (min:sec) 3:12 Cumulative time (S) Moles of S2082-reacted 1 192 2.0 x 10+ 2 3.18 390 4.0 x 104 3:23 3 6.0 x 10-4 593 foa lol 3.29 3:29 4 8.0 x 104 5 10.0 x 104 6 Idha 3:30 3:41 12.0 x 10-4 1450 7 14.0 x 104
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


