Answered step by step
Verified Expert Solution
Question
1 Approved Answer
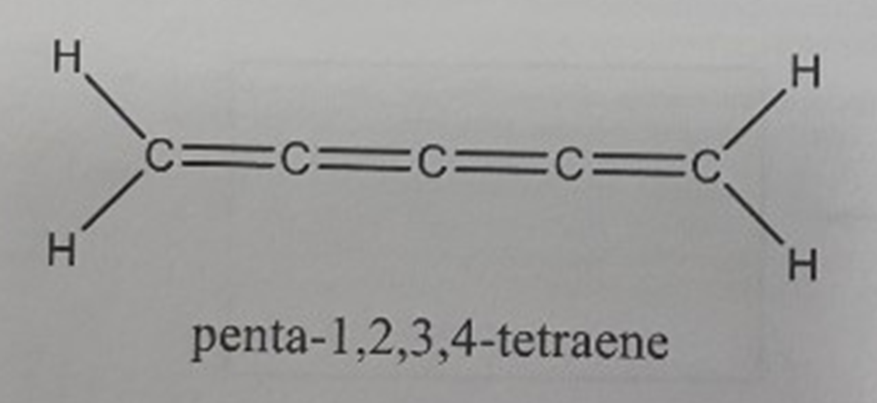
Draw a 3 D representation of the molecule penta - 1 , 2 , 3 , 4 - tetraene using the following EXPLICIT instructions: sigma
Draw a D representation of the molecule pentatetraene using the following EXPLICIT instructions: sigma bonds in the plane of the paper are normal lines, sigma bonds or orbitals projecting in front of the page are heavy lines, sigma bonds or orbitals projecting behind the page are dashed lines, and pi bonds are shown by drawing the lobes of the porbitals and indicating the overlaps. What is the hybridization of each atom except H in this molecule?pentatetraene
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


