Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Draw a labeled, schematic of the process, indicating all inlet/outlet streams and conditions Determine the value of the equilibrium constant at the conditions of the
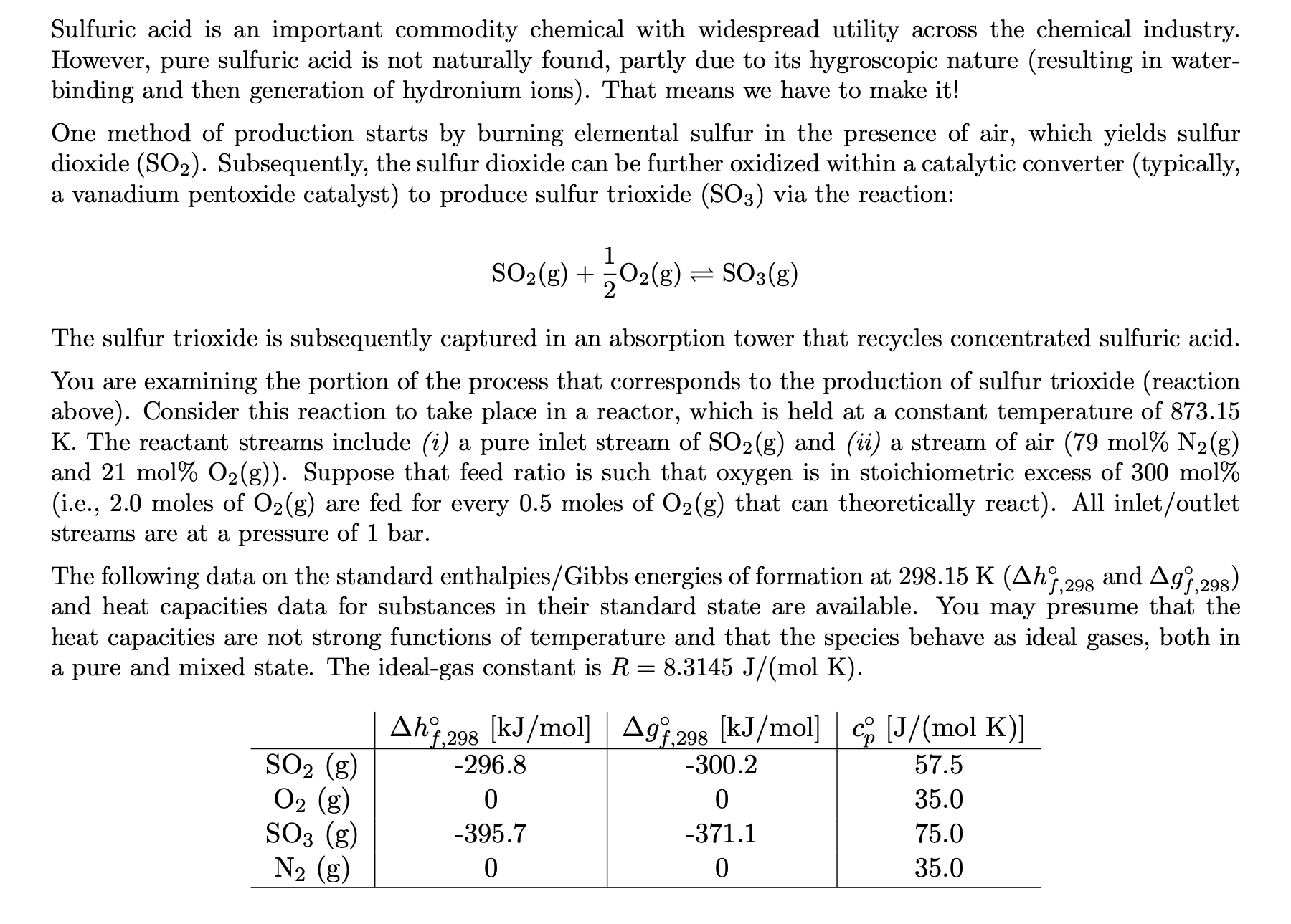
- Draw a labeled, schematic of the process, indicating all inlet/outlet streams and conditions
- Determine the value of the equilibrium constant at the conditions of the reactor. (If you are unable to obtain an answer for this part and require one for subsequent parts, presume that K = 10.)
- Write expressions for the composition of the reactor components (SO2, SO3, O2, and N2) using a basis of 1 mol SO2. Using any reasonable or necessary approximations, obtain an expression for the equilibrium constant as a function of the extent of reaction . You do not need a numerical result.
- Suppose that the composition of the exit stream is measured to be yso3= 0.06. Assess whether you think the reaction has proceeded to equilibrium if the equilibrium extent of reaction is eq 0.803.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


