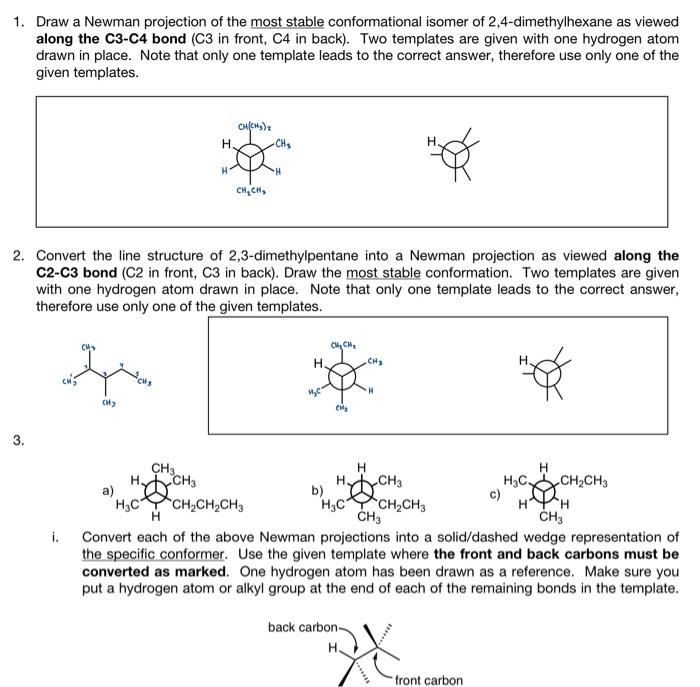
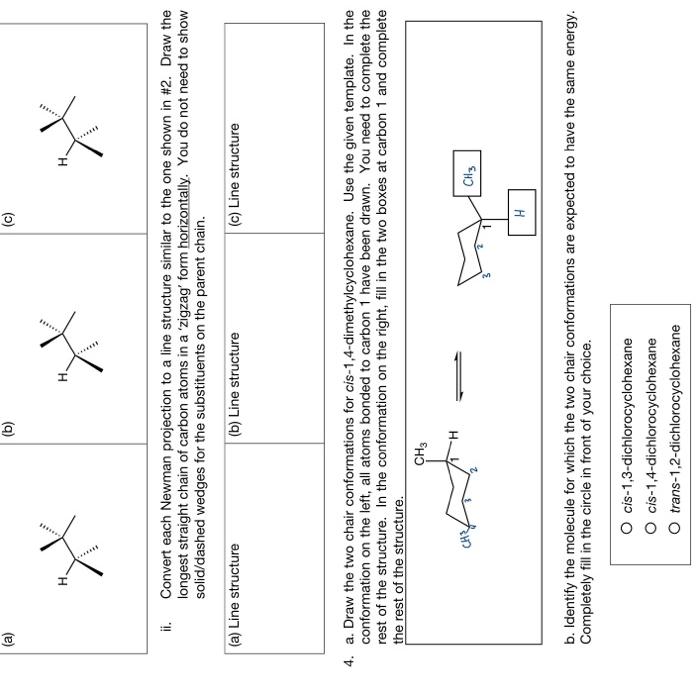
Draw a Newman projection of the most stable conformational isomer of 2,4-dimethylhexane as viewed along the C3-C4 bond (C3 in front, C4 in back). Two templates are given with one hydrogen atom drawn in place. Note that only one template leads to the correct answer, therefore use only one of the given templates. Convert the line structure of 2,3-dimethylpentane into a Newman projection as viewed along the C.-C.2 hond (C.O in front C.2 in hark) nraw the mnet etahle confnrmation Two temnlates are aiven I. Lonvert eacn or tne adove ivewman projections into a solla/aasned weage representation or the specific conformer. Use the given template where the front and back carbons must be converted as marked. One hydrogen atom has been drawn as a reference. Make sure you put a hydrogen atom or alkyl group at the end of each of the remaining bonds in the template. ii. Convert each Newman projection to a line structure similar to the one shown in \#2. Draw the longest straight chain of carbon atoms in a 'zigzag' form horizontally. You do not need to show solid/dashed wedges for the substituents on the parent chain. 4. a. Draw the two chair conformations for cis-1,4-dimethylcyclohexane. Use the given template. In the conformation on the left, all atoms bonded to carbon 1 have been drawn. You need to complete the rest of the structure. In the conformation on the right, fill in the two boxes at carbon 1 and complete the rest of the structure b. Identify the molecule for which the two chair conformations are expected to have the same energy. Completely fill in the circle in front of your choice. cis-1,3-dichlorocyclohexane cis-1,4-dichlorocyclohexane trans-1,2-dichlorocyclohexane Draw a Newman projection of the most stable conformational isomer of 2,4-dimethylhexane as viewed along the C3-C4 bond (C3 in front, C4 in back). Two templates are given with one hydrogen atom drawn in place. Note that only one template leads to the correct answer, therefore use only one of the given templates. Convert the line structure of 2,3-dimethylpentane into a Newman projection as viewed along the C.-C.2 hond (C.O in front C.2 in hark) nraw the mnet etahle confnrmation Two temnlates are aiven I. Lonvert eacn or tne adove ivewman projections into a solla/aasned weage representation or the specific conformer. Use the given template where the front and back carbons must be converted as marked. One hydrogen atom has been drawn as a reference. Make sure you put a hydrogen atom or alkyl group at the end of each of the remaining bonds in the template. ii. Convert each Newman projection to a line structure similar to the one shown in \#2. Draw the longest straight chain of carbon atoms in a 'zigzag' form horizontally. You do not need to show solid/dashed wedges for the substituents on the parent chain. 4. a. Draw the two chair conformations for cis-1,4-dimethylcyclohexane. Use the given template. In the conformation on the left, all atoms bonded to carbon 1 have been drawn. You need to complete the rest of the structure. In the conformation on the right, fill in the two boxes at carbon 1 and complete the rest of the structure b. Identify the molecule for which the two chair conformations are expected to have the same energy. Completely fill in the circle in front of your choice. cis-1,3-dichlorocyclohexane cis-1,4-dichlorocyclohexane trans-1,2-dichlorocyclohexane