Question
Draw an alternative Lewis structure for CH2Br. I can't seem toget this right :( Determine the formal charge on the bromine atom in the following
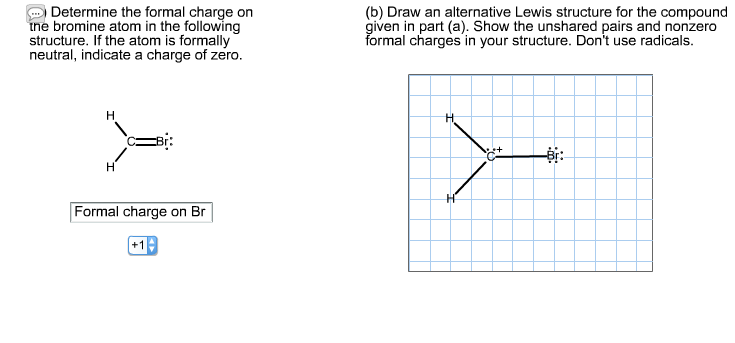
Draw an alternative Lewis structure for CH2Br. I can't seem toget this right :(
Determine the formal charge on the bromine atom in the following structure. If the atom is formally neutral, indicate a charge of zero. H - H Formal charge on Br +19 (b) Draw an alternative Lewis structure for the compound given in part (a). Show the unshared pairs and nonzero formal charges in your structure. Don't use radicals. H H C+
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
Bromine has 7 valence electrons It forms 2 bonds with its adjacent atoms Hence the number of b...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: L. G. Wade Jr.
8th edition
321768418, 978-0321768414
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



