Answered step by step
Verified Expert Solution
Question
1 Approved Answer
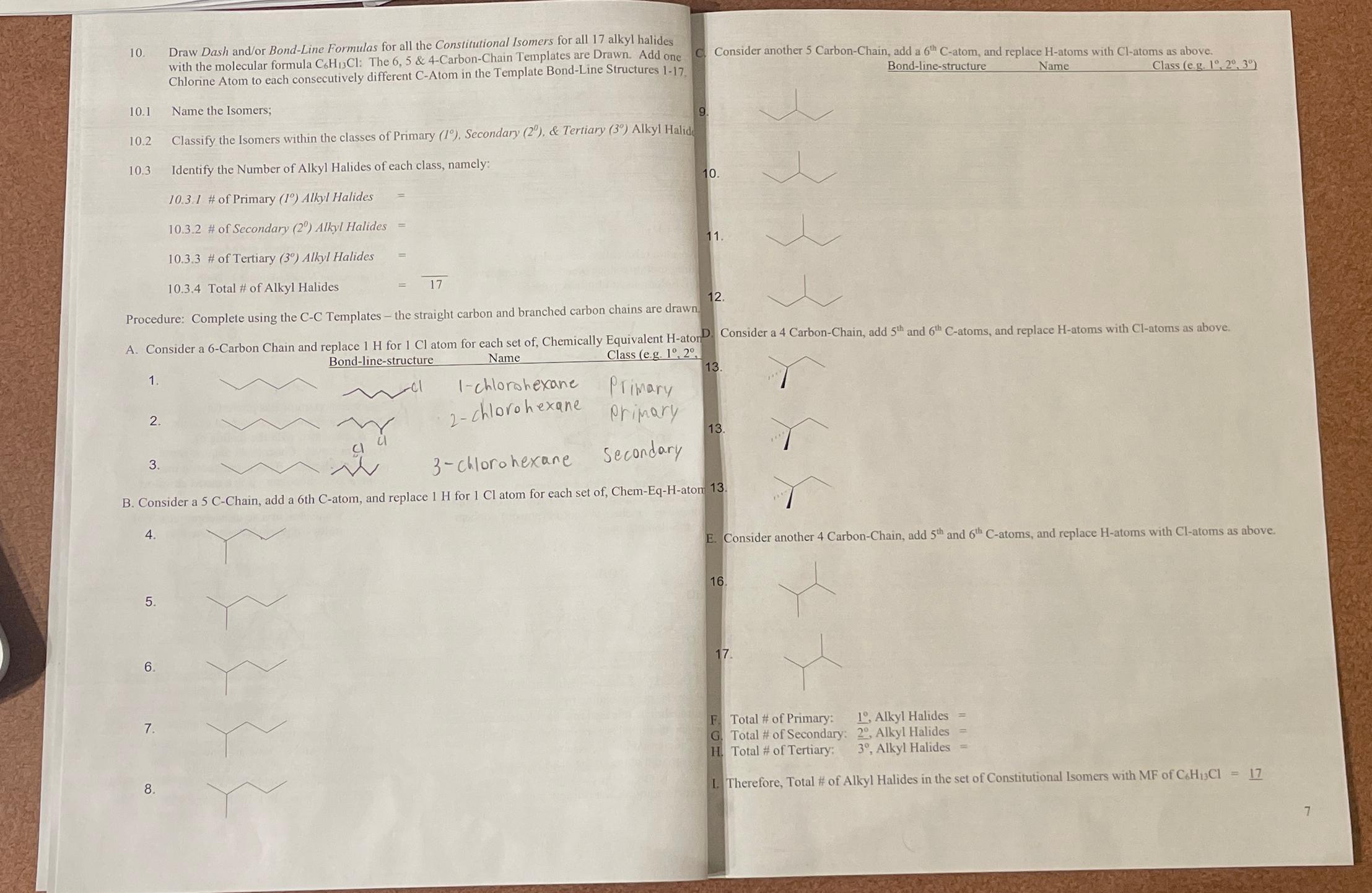
Draw Dash and / or Bond - Line Formulas for all the Constitutional Isomers for all 1 7 alkyl halides with the molecular formula C
Draw Dash andor BondLine Formulas for all the Constitutional Isomers for all alkyl halides with the molecular formula : The & CarbonChain Templates are Drawn. Add one Chlorine Atom to each consecutively different CAtom in the Template BondLine Structures
Name the Isomers;
Classify the Isomers within the classes of Primary Secondary & Tertiary Alkyl Halid
Identify the Number of Alkyl Halides of each class, namely:
# Primary Alkyl Halides
# Secondary Alkyl Halides
# Tertiary Alkyl Halides
Total # Alkyl Halides
Procedure: Complete using the CC Templates the straight carbon and branched carbon chains are drawn.
A Consider a Carbon Chain and replace for atom for each set of Chemically Equivalent aton Bondlinestructure
Name Class eg
chlorohexane Primary chlorohexane Pripary
obracechlorohexane secondary Bondlinestructure
Name
Class eg:
Consider a CarbonChain, add and atoms, and replace atoms with atoms as above.
B Consider a Chain, add a th atom, and replace for atom for each set of ChemEqHatom
F Total # of Primary: Alkyl Halides
G Total # of Secondary: Alkyl Halides
H Total # of Tertiary: Alkyl Halides
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


