driving CSTR only.
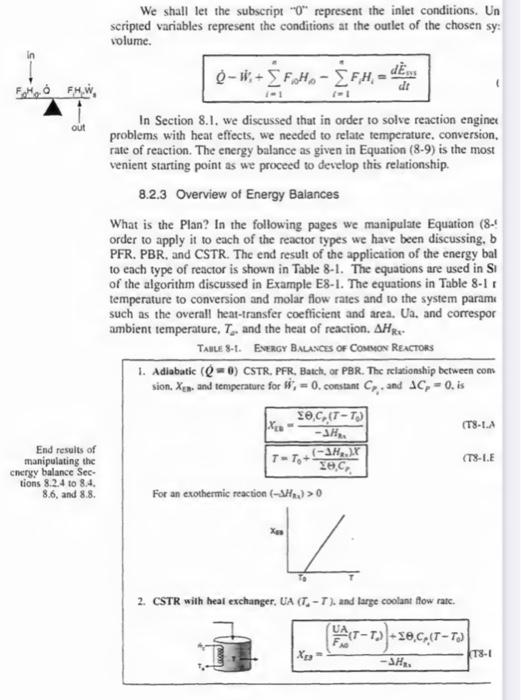
We shall let the subscript 0 represent the inlet conditions. Un scripted variables represent the conditions at the outlet of the chosen sy: volume. QWi+i=1nFi0H0i=1nFiHi=dtdE^By In Section 8.1. we discussed that in order to solve reaction enginet problems with heat effects, we needed to relate temperature, conversion. rate of reaction. The energy balance as given in Equation (8-9) is the most venient starting point as we proceed to develop this relationship. 8.2.3 Overview of Energy Balances What is the Plan? In the following pages we manipulate Equation (8.t order to apply it to each of the reactor types we have been discussing, b PFR. PBR, and CSTR. The end result of the application of the energy bal to each type of reactor is shown in Table 8-1. The equations are used in S of the algorithm discussed in Example E8-1. The equations in Table 8-1 r temperature to conversion and molar flow rates and to the system param. such as the overall heat-transfer coefticient and area. Ua. and correspor ambient temperature, Ta. and the heat of reaction. Hkx. Talle 8-1. Evergy Bnchaces of Connon Reactons 1. Adiabatic (Q=) CSTR, PFR, Batch, or PBR. The relationship between cons sion, Xta., and temperature for Hi=0, constant CPi, and CP=0, is (181.4 End results of thanipulating the cnergy balance Sections 8.2 .4 to 8.4 . 8.6 , and 8.8 . For an evothermic reaction (S//43)>0 2. CSTR wilh heat exchanger, UA (T4T), and large coolant flow ratc. Sec. 5.2 the Energy balinoe 475 We see that the units for flow work are consistent with the other terms in Equation (8-2), i.e., 3/5. In most instances, the flow work term is combined with those terms in the energy balance that represent the energy exchange by mass flow across the system boundaries. Substituting Equation (8-4) into (8-3) and grouping terms, we have dtdE^so=QWi+i=1nFi(E1+PV~ii=1ni=1nFi(Ei+PV~jout The energy Ei is the sum of the internal energy (Ui), the kinetic energy (ui2/2), the potential energy (gz1), and any other energies, such as electric or magnetic energy or light; Ei=Ui+2ui2+gzi+other In aimost all chemical reactor situations, the kinetic, potential, and "other" energy lerms are negligible in comparison with the enthalpy, heat transfer, and work terms, and hence will be omitied: that is. Ei=Ui We recall that the enthalpy, Hi(J/mol), is defined in terms of the internal energy Ui(J/mol), and the product PVi(1Pam3/mol=1J/mol) : Hi=Ui+PVi Typical units of Hf are (Hi)=moliJorlbmoliBruormolical Enthalpy carried into (or out of) the system can be expressed as the sum of the net internal energy carried into (or out of) the system by mass flow plus the flow work: FiHi=Fi(Ui+PVi) Combining Equations (8-5), (8-7), and (88), we can now write the energy belance in the form dtdE^ws=QHi+i=1FiHii=1i=1mFiHian The energy of the system at any instant in time, E~mo. is the sum of the products of the number of moles of each species in the system multiplied by their respective energies. This term will be discussed in more detail when unsteady-state reactor operation is considered in Chapter 9. We will assume the contents of the system volume are well mixed, an assumption that we could relax but that would require a couple pages of text to develop. and the end result would be the same! The unsteady-state energy balance for an open well-mixed system that has n species. cach entering and leaving the system at their respective molar flow rates Fj (moles of i per time) and with their respective energy Ei (joules per mole of i ), is dtdEus=Qj+i=1nEiFii=1nEiFiour We will now discuss each of the terms in Equation (8-3). 8.2.2 Evaluating the Work Term It is customary to separate the work term. Wr. into flow work and other work. W5. The term W,, often referred to as the shaft work, could be produced from such things as a stirrer in a CSTR or a turbinc in a PFR. Flow werk is work that is necessary to get the mass iato and out of the system. For exanple. when shear stresses are absent. we write [Rate of flow work] Flow work and shaft work H^=i=1nF,PVii=1n+i=1nFiPViiut[Rateofflowwork]+Ws where P is the pressure (Pa)[1Pa=1 Newton/ /m2=1kgm/s2/m2] and Vis is the specific molar volume of species i(m1/mol of i. Let's look at the units of the flow work term. which is F,PV, where Fi is in mol/s, P is Pa(1Pa=1 Newton/m), and V^1 is m3/mol. FiPVi[=1smolm2Newtonmolm3=(Newtonm)s1=Joulcs/s=Watts Sec, 8.2 The Energy Balance 475 We see that the units for flow work are consistent with the other terms in Equation (8-2), i.e., J/s. In most instances, the flow work term is combined with those terms in the energy balance that represent the energy exchange by mass flow across the system boundaries. Substituting Equation (8-4) into (83) and grouping terms, we have dtdEsi=QHi+nFi(Ei+PVi)nFi(Ei+PVi)