Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Dry air is used as the O2 source in a combustion reaction. The conversion rate (X) of O2 during the reaction and the reaction rates
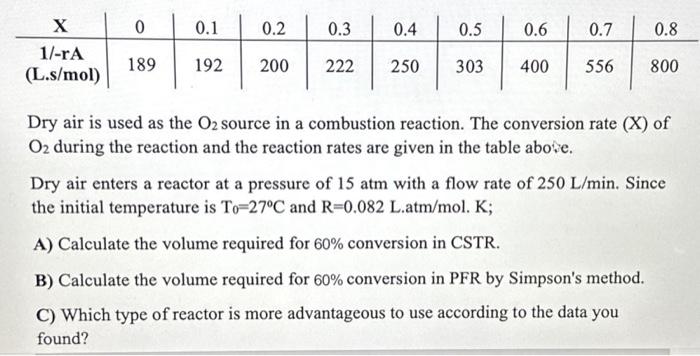
Dry air is used as the O2 source in a combustion reaction. The conversion rate (X) of O2 during the reaction and the reaction rates are given in the table above. Dry air enters a reactor at a pressure of 15 atm with a flow rate of 250 L/min. Since the initial temperature is To-27C and R=0.082 L.atm/mol. K;
A) Calculate the volume required for 60% conversion in CSTR.
B) Calculate the volume required for 60% conversion in PFR by Simpson's method.
C) Which type of reactor is more advantageous to use according to the data you found?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


