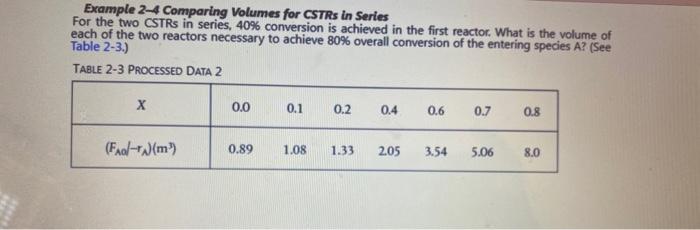
duction to Chemical Reactor Design Problem 6: Elements of Chemical Reaction Engineering 6 Ed P2-2 (b), (c) and (e) For part (b) do not attempt for the doubled flow rate 022 P2-2A a. Revisit the data in Table 2-1 Row Data and calculate the batch reactor (BR) times to achieve 10% 50%, and 80% conversion when 100 moles of A are charged to a 400 dm reactor b. Revisit Examples 2-1 through 2-3. How would your answers change if the flow rate. Fi were cut in half? If it were doubled? What conversion can be achieved in a 4.5 m PFR and in a 4.5 m CSTR? c. Revisit Example 2-2. Being a company about to go bankrupt, you can only afford a 25 m CSTR What conversion can you achieve? d. Revisit Example 2-3. What conversion could you achieve if you could convince your boss. De Pennypincher to spend more money to buy a l mPFR to attach to a 240 m CSTR? e. Revisit Example 2-4. How would your answers change if the two CSTRS (one 0.82 m and the other 3.2 m) were placed in parallel with the flow. Fas divided equally between the reactors? f. Revisit Example 2-5. (1) What is the worst possible way to arrange the two CSTRs and one PFR? (2) What would be the reactor volumes if the two intermediate conversions were changed to 20% and 50%, respectively? (3) What would be the conversions. XX and X, if all the reactors had the same volume of 100 dm and were placed in the same order? X dx 9. Revisit Example 2-6. If the term Col is 2 seconds for 30% conversion how much fluid (m/min) can you process in a 3 m reactor? LA Example 2-2 Sizing a PFR The reaction described by the data in Tables 2-1 and 2-2 is to be carried out in a PFR. The entering molar flow rate of A is again, Fe = 0.4 mol/s. a. First, use one of the integration formulas given in Appendix A.4 to determine the PFR reactor volume necessary to achieve 80% conversion. b. Next, shade the area in Figure 2-2(b) that would give the PFR volume necessary to achieve 80conversion. c. Finally, make a qualitative sketch of the conversion X and the rate of reaction -TA down the length (volume) of the reactor. Example 2-1 Sizing a CSTR The reaction described by the data in Table 2-2 A-B is to be carried out in a CSTR. Species A enters the reactor at a molar flow rate of Fx = 0.4 mol which is the flow rate used to construct the Levenspiel plot in Figure 2-2(b). a. Using the data in either Table 2-2 or Figure 2-2(b), calculate the volume necessary to achieve 80% conversion in a CSTR. b. Shade the area in Figure 2-2(b) that would give the CSTR volume necessary to achieve 80% 5 conversion. Example 2-4 Comparing Volumes for CSTRs in Series For the two CSTRs in series, 40% conversion is achieved in the first reactor. What is the volume of each of the two reactors necessary to achieve 80% overall conversion of the entering species A? (See Table 2-3.) TABLE 2-3 PROCESSED DATA 2 0.0 0.1 0.2 0.4 0.6 0.7 0.8 (Faal- )(m) 0.89 1.08 1.33 2.05 3.54 5.06 8.0 duction to Chemical Reactor Design Problem 6: Elements of Chemical Reaction Engineering 6 Ed P2-2 (b), (c) and (e) For part (b) do not attempt for the doubled flow rate 022 P2-2A a. Revisit the data in Table 2-1 Row Data and calculate the batch reactor (BR) times to achieve 10% 50%, and 80% conversion when 100 moles of A are charged to a 400 dm reactor b. Revisit Examples 2-1 through 2-3. How would your answers change if the flow rate. Fi were cut in half? If it were doubled? What conversion can be achieved in a 4.5 m PFR and in a 4.5 m CSTR? c. Revisit Example 2-2. Being a company about to go bankrupt, you can only afford a 25 m CSTR What conversion can you achieve? d. Revisit Example 2-3. What conversion could you achieve if you could convince your boss. De Pennypincher to spend more money to buy a l mPFR to attach to a 240 m CSTR? e. Revisit Example 2-4. How would your answers change if the two CSTRS (one 0.82 m and the other 3.2 m) were placed in parallel with the flow. Fas divided equally between the reactors? f. Revisit Example 2-5. (1) What is the worst possible way to arrange the two CSTRs and one PFR? (2) What would be the reactor volumes if the two intermediate conversions were changed to 20% and 50%, respectively? (3) What would be the conversions. XX and X, if all the reactors had the same volume of 100 dm and were placed in the same order? X dx 9. Revisit Example 2-6. If the term Col is 2 seconds for 30% conversion how much fluid (m/min) can you process in a 3 m reactor? LA Example 2-2 Sizing a PFR The reaction described by the data in Tables 2-1 and 2-2 is to be carried out in a PFR. The entering molar flow rate of A is again, Fe = 0.4 mol/s. a. First, use one of the integration formulas given in Appendix A.4 to determine the PFR reactor volume necessary to achieve 80% conversion. b. Next, shade the area in Figure 2-2(b) that would give the PFR volume necessary to achieve 80conversion. c. Finally, make a qualitative sketch of the conversion X and the rate of reaction -TA down the length (volume) of the reactor. Example 2-1 Sizing a CSTR The reaction described by the data in Table 2-2 A-B is to be carried out in a CSTR. Species A enters the reactor at a molar flow rate of Fx = 0.4 mol which is the flow rate used to construct the Levenspiel plot in Figure 2-2(b). a. Using the data in either Table 2-2 or Figure 2-2(b), calculate the volume necessary to achieve 80% conversion in a CSTR. b. Shade the area in Figure 2-2(b) that would give the CSTR volume necessary to achieve 80% 5 conversion. Example 2-4 Comparing Volumes for CSTRs in Series For the two CSTRs in series, 40% conversion is achieved in the first reactor. What is the volume of each of the two reactors necessary to achieve 80% overall conversion of the entering species A? (See Table 2-3.) TABLE 2-3 PROCESSED DATA 2 0.0 0.1 0.2 0.4 0.6 0.7 0.8 (Faal- )(m) 0.89 1.08 1.33 2.05 3.54 5.06 8.0