Question
A sample of water at 20.3 C required an input of 1.78 x 104 J of heat to reach its boiling point, 100.0 C.
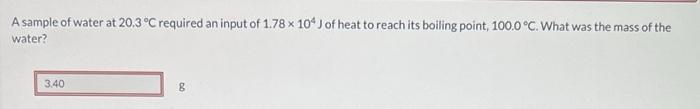
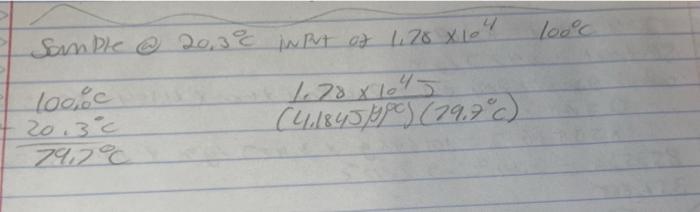
A sample of water at 20.3 C required an input of 1.78 x 104 J of heat to reach its boiling point, 100.0 C. What was the mass of the water? 3.40 8 Sample @ 20,3C IN Put of 1,70 X 10 100%c 20.3C 79.7C 11.781045 (4.1845/P) (79.7c) loo
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Managerial Accounting
Authors: Ray H. Garrison, Eric W. Noreen, Peter C. Brewer
13th Edition
978-0073379616, 73379611, 978-0697789938
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



