Answered step by step
Verified Expert Solution
Question
1 Approved Answer
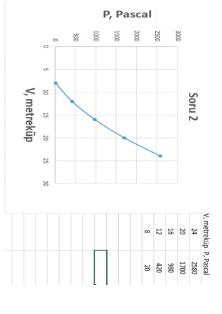
During the gas expansion process in a frictionless piston assembly, the gas pressure changes from 2580 Pascal to 20 Pascal value as P= aV^2+b depending
During the gas expansion process in a frictionless piston assembly, the gas pressure changes from 2580 Pascal to 20 Pascal value as P= aV^2+b depending on V. Here a and b are the experimental constant coefficients. Initially, the volume of the gas was found to be 24m^3 and in the final case the volume of the gas was 8m3. Then this gas is compressed to a pressure of 545 PA. With the same conditions valid, answer the following questions regarding the compression process. A) what is its volume in the final state, m^3 B) How many joules is the compression work done to the system according to hook notation? C) What is the entropy change of the gas during this process? D ) Find the coefficients a and b .V, metrekup P, Pracal \begin{tabular}{|c|c|} \hline 24 & 258 \\ \hline 22 & 1300 \\ \hline 15 & 950 \\ \hline 12 & 420 \\ \hline 8 & 20 \\ \hline \end{tabular} V, metrekup P, Pracal \begin{tabular}{|c|c|} \hline 24 & 258 \\ \hline 22 & 1300 \\ \hline 15 & 950 \\ \hline 12 & 420 \\ \hline 8 & 20 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started