Answered step by step
Verified Expert Solution
Question
1 Approved Answer
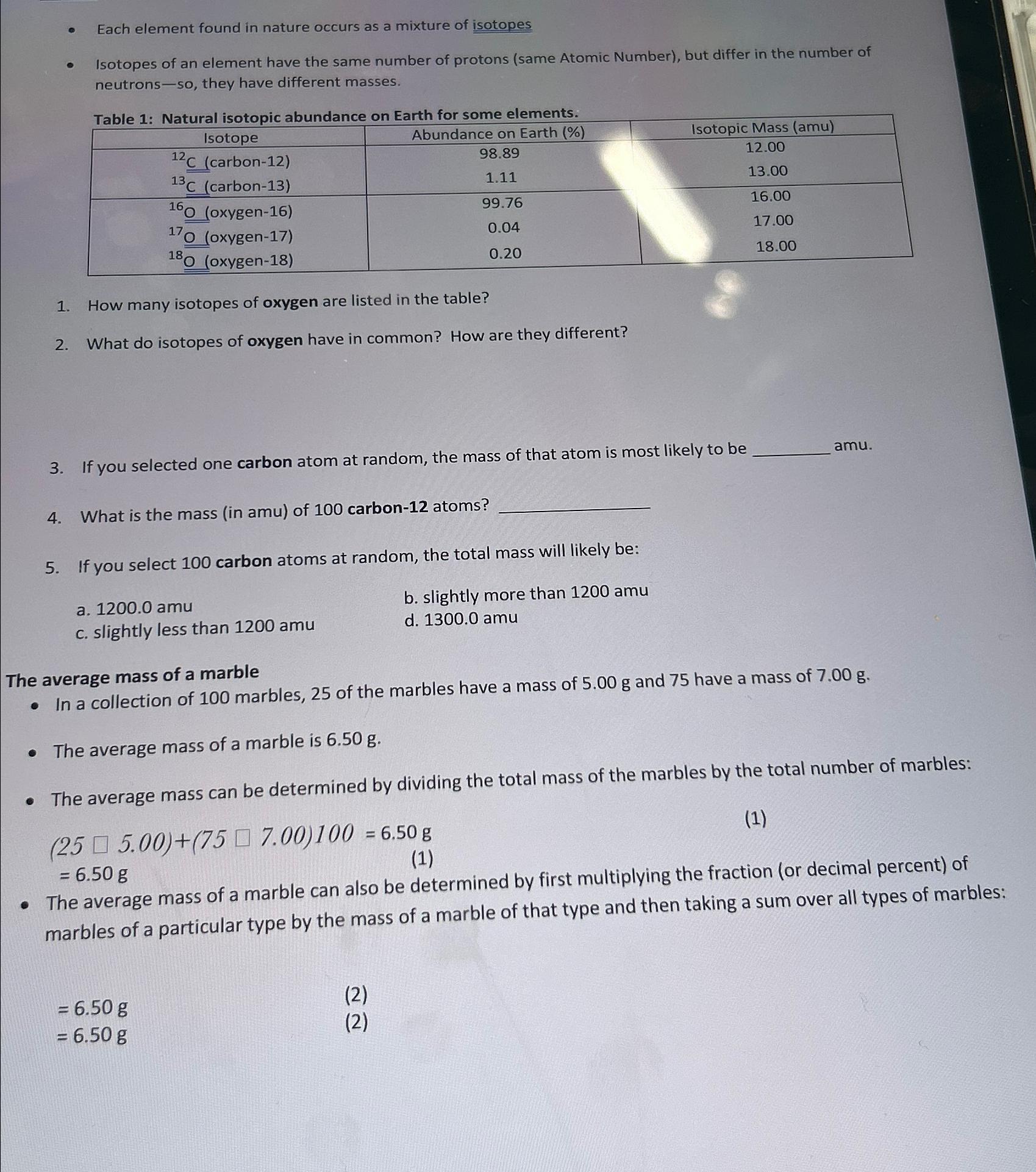
Each element found in nature occurs as a mixture of isotopes Isotopes of an element have the same number of protons ( same Atomic Number
Each element found in nature occurs as a mixture of isotopes
Isotopes of an element have the same number of protons same Atomic Number but differ in the number of neutrons so they have different masses.
Table : Natural isotopic abundance on Earth for some elements.
tableIsotopeAbundance on Earth Isotopic Mass amucarboncarbonoxygen O oxygenoxygen
How many isotopes of oxygen are listed in the table?
What do isotopes of oxygen have in common? How are they different?
If you selected one carbon atom at random, the mass of that atom is most likely to be amu.
What is the mass in amu of carbon atoms?
If you select carbon atoms at random, the total mass will likely be:
a
b slightly more than
c slightly less than
d
The average mass of a marble
In a collection of marbles, of the marbles have a mass of and have a mass of
The average mass of a marble is
The average mass can be determined by dividing the total mass of the marbles by the total number of marbles:
The average mass of a marble can also be determined by first multiplying the fraction or decimal percent of marbles of a particular type by the mass of a marble of that type and then taking a sum over all types of marbles:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


