Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the following data on 20 chemical reactions, with Y= chromatographic retention time (seconds) and X= molecular weight (gm/mole). Retention Time and Molecular Weight
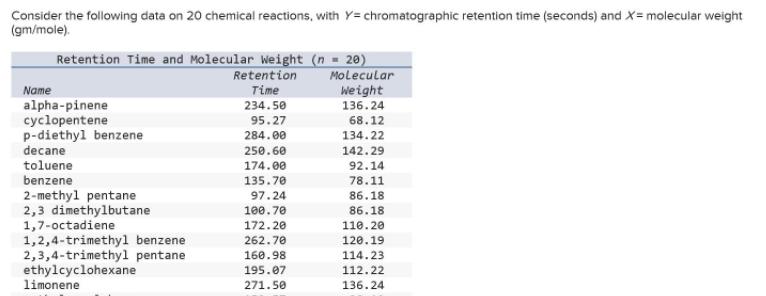
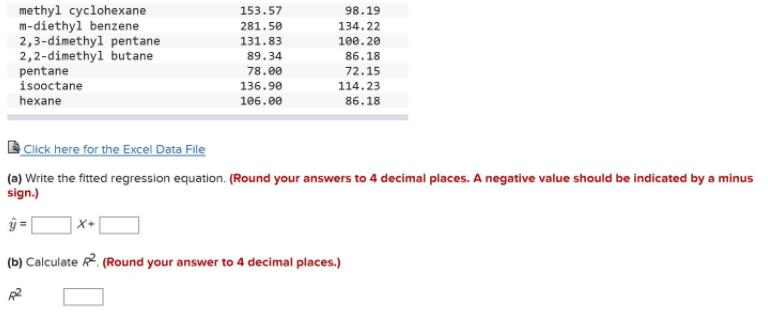
Consider the following data on 20 chemical reactions, with Y= chromatographic retention time (seconds) and X= molecular weight (gm/mole). Retention Time and Molecular Weight (n = 20) Molecular Weight Retention Name Time alpha-pinene cyclopentene p-diethyl benzene decane toluene benzene 234.50 136.24 95.27 68.12 284.00 134.22 250.60 142.29 174.00 92.14 135.70 78.11 2-methyl pentane 2,3 dimethylbutane 1,7-octadiene 1,2,4-trimethyl benzene 2,3,4-trimethyl pentane ethylcyclohexane limonene 97.24 86.18 100.70 86.18 172.20 110.20 262.70 120.19 114.23 112.22 160.98 195.07 271.50 136.24 methyl cyclohexane m-diethyl benzene 2,3-dimethyl pentane 2,2-dimethyl butane pentane isooctane 153.57 98.19 281.50 134.22 131.83 100.20 89.34 86.18 78.00 72.15 136.90 114.23 hexane 106.00 86.18 Click here for the Excel Data File (a) Write the fitted regression equation. (Round your answers to 4 decimal places. A negative value should be indicated by a minus sign.) (b) Calculate R. (Round your answer to 4 decimal places.)
Step by Step Solution
★★★★★
3.50 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Answer x y xx yy xxyy 13624 2345 9513832 40857664 1971580 6812 9527 13894629 56715961 2807218 13422 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


