Question
Based on the single-step global mechanism given by Eqns. 5.1 and 5.2 (page 156), write down the chemical reaction for octane combustion at equivalence

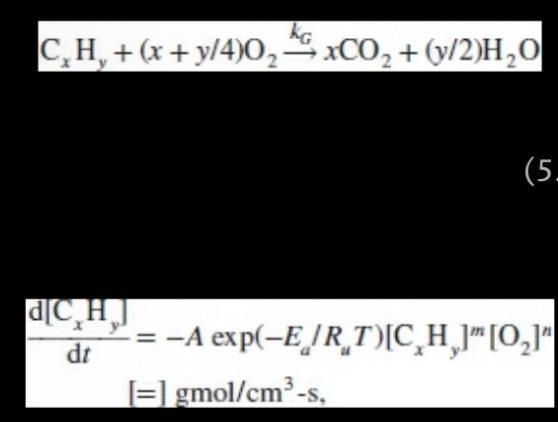
Based on the single-step global mechanism given by Eqns. 5.1 and 5.2 (page 156), write down the chemical reaction for octane combustion at equivalence ratio of 1.0 and then determine the molar concentrations of octane and oxygen in units of gmol/cm. CH + (x + y/4)0xCO+ (v/2)HO d[CH] y dt (5. -=-A exp(-E/RT)[CH] [0]" [=] gmol/cm-s,
Step by Step Solution
3.49 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
To obtain the molar concentration of octane and oxygen in gmolcm 3 for the combustion of octane C 8 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Management and Cost Accounting
Authors: Colin Drury
8th edition
978-1408041802, 1408041804, 978-1408048566, 1408048566, 978-1408093887
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



