Question
Electrostatic and gravitational forces. The average distance r between the electron and the central proton in the hydrogen atom is 5.3 x 10-11 m.
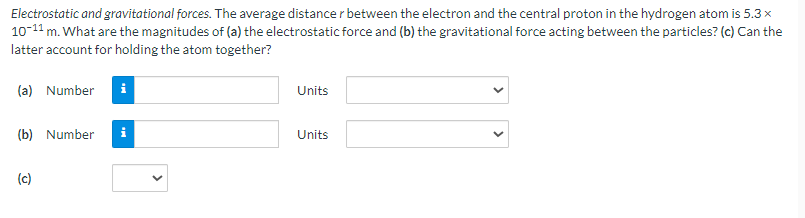
Electrostatic and gravitational forces. The average distance r between the electron and the central proton in the hydrogen atom is 5.3 x 10-11 m. What are the magnitudes of (a) the electrostatic force and (b) the gravitational force acting between the particles? (c) Can the latter account for holding the atom together? (a) Number (b) Number i (c) Units Units
Step by Step Solution
3.37 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
a To calculate the magnitude of the electrostatic force between the electron and the central proton ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Applied Physics
Authors: Dale ewen, Neill schurter, P. erik gundersen
10th Edition
978-0136116332, 136116337, 9780132830096, 978-0132109277
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



