Answered step by step
Verified Expert Solution
Question
1 Approved Answer
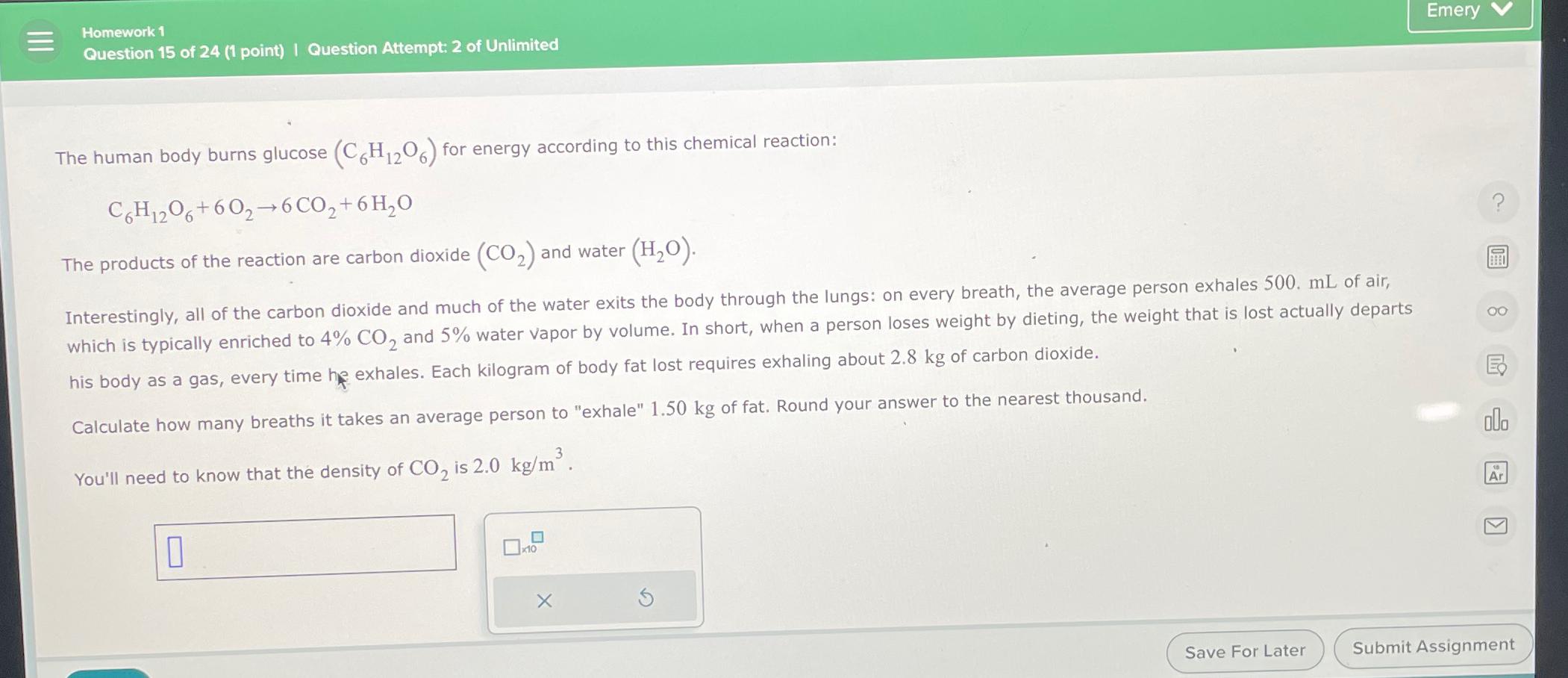
Emery Homework 1 Question 1 5 of 2 4 ( 1 point ) | Question Attempt: 2 of Unlimited The human body burns glucose (
Emery
Homework
Question of point Question Attempt: of Unlimited
The human body burns glucose for energy according to this chemical reaction:
The products of the reaction are carbon dioxide and water his body as a gas, every time he exhales. Each kilogram of body fat lost requires exhaling about of carbon dioxide.
Calculate how many breaths it takes an average person to "exhale" of fat. Round your answer to the nearest thousand.
You'll need to know that the density of is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


