Answered step by step
Verified Expert Solution
Question
1 Approved Answer
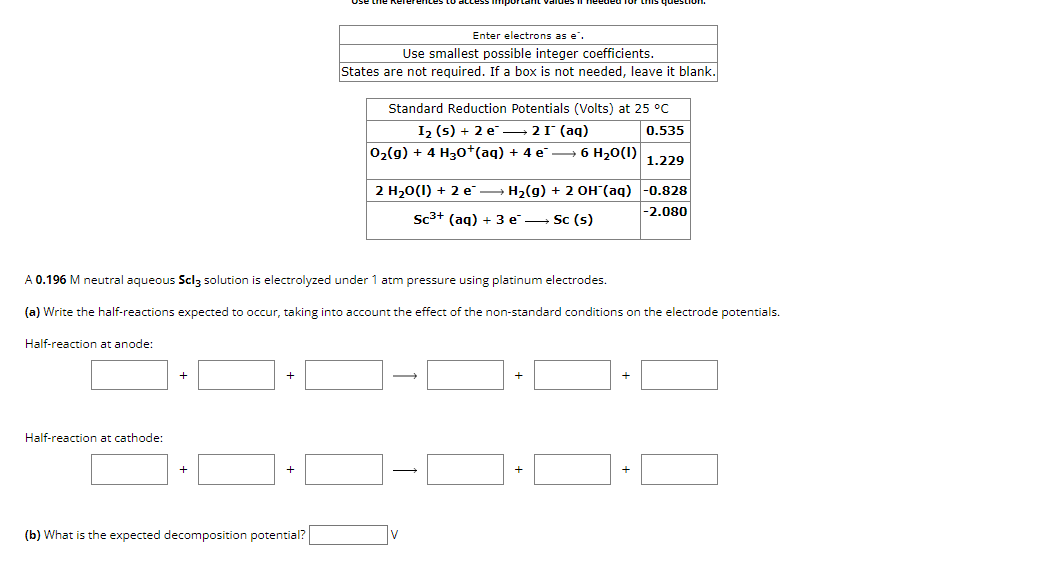
Enter electrons as e * * . Use smallest possible integer coefficients. States are not required. If a box is not needed, leave it blank.
Enter electrons as
Use smallest possible integer coefficients.
States are not required. If a box is not needed, leave it blank.
A neutral aqueous solution is electrolyzed under atm pressure using platinum electrodes.
a Write the halfreactions expected to occur, taking into account the effect of the nonstandard conditions on the electrode potentials.
Halfreaction at anode:
Halfreaction at cathode:
b What is the expected decomposition potential?
IV
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


