Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Enter your answer in the provided box. The thermal decomposition of ethylene occurs in many industrial contexts: during ethylene transit in pipelines, formation of polyethylene,
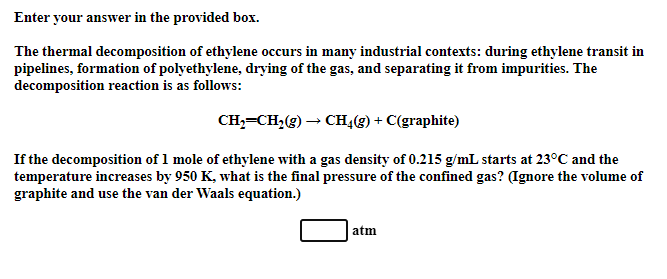
Enter your answer in the provided box.
The thermal decomposition of ethylene occurs in many industrial contexts: during ethylene transit in
pipelines, formation of polyethylene, drying of the gas, and separating it from impurities. The
decomposition reaction is as follows:
If the decomposition of mole of ethylene with a gas density of starts at and the
temperature increases by what is the final pressure of the confined gas? Ignore the volume of
graphite and use the van der Waals equation.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


