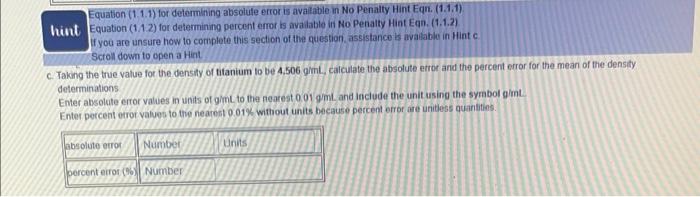
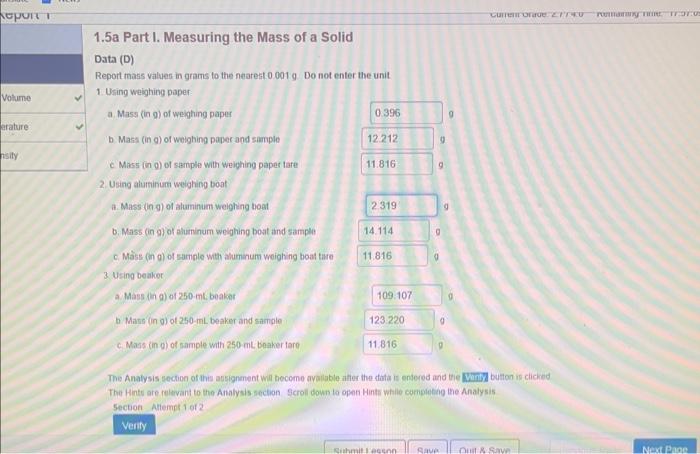
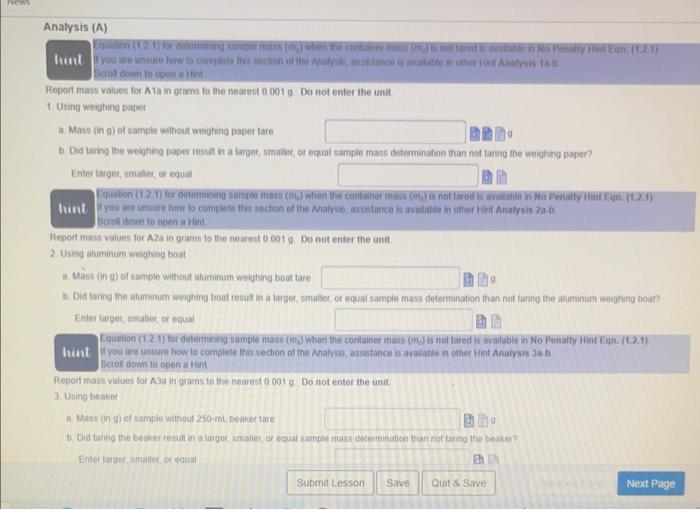
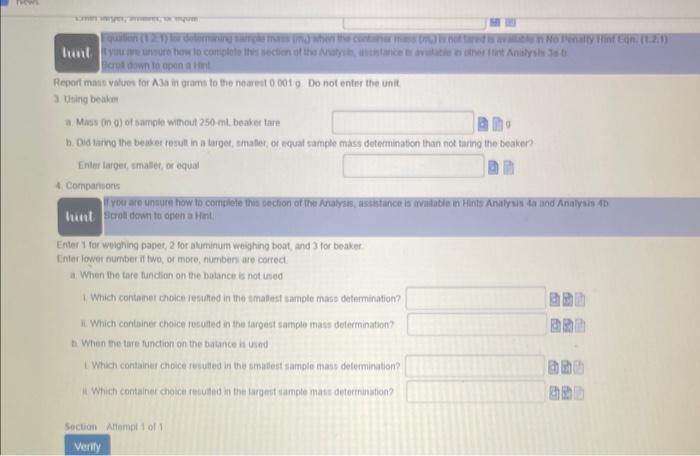
Equation (1.1.1) for determining absolufe error is available in No Penaity Hint Eqr. (1.1,1) Whint Equation (1.1.2) for determining percent enor is available in No Penatty Hint Eqn. (1.1.2) If you are unsure how to conplete this section of the question. assistance bs avalable in Hint c Scroll down to open a Hint. c. Taking the true value for the densily of titanium to be 4.506ginh. calculate the absolute etror and the percent error for the mean of the density determinations Enter absolute error values in units of gimL to the nearest 0.019mL and include the unit using the symbol gimt. Enter percent errof values to the nearest 0.01% without units because percent efrof are unitless quantitins. 1.5a Part I. Measuring the Mass of a Solid Data (D) Report mass values in grams to the nearest 0.0019 Do not enter the unit The Analysis section of ahis assignenent wir become avalibble itter the ditati is engered and the button is cliched The Hinte are relivant to the Allalysis section Scrol down to open Hints whilo completing the Analysis 5ection Aliempt 1 of 2 ficret down to open a Hint. Report mass values for A1a in grams to the nearest 0.001g. Do not enter the unit 1. Using weighing paper a. Mass (in 0) of sample winout weighing papes tare b. Did taring the woighing paper rusult in a larges, amable, or equat sample mass determination than not tarng the waighing papen? Enter laiget, smalies, or equat 11 you are unsure how lo comblele this section of the Anatysi, assintance is avalabie in other tirt Anatysis za b. Scobit down to open a Hint Report mass values for A a in grams to tie nearest 00019 Do not enter the unit 2. Using aluminum weighung boat a. Mast (in g) of sampie without aluminum weighing boat tare b. Did taring the aluminam weighing boat resut in a larger, smasec, of equal sample mass defermination than not taring the aluminum woighing boar? Entor larges, imatten, or equal Equation (1.21) for determining sample mass (mi) when the container mast (imi) is not tared is wailiabie in No Penaty Hint Eqn. (t.2.1). If you are unsure how to complete this section of the Analyso, assistance is avaiabie in oher Hint Analysis Ja .b. sccoll dowr to open a Hint Fepon maso values for A3a in grams to the nearest 00019 . Do not enter the unit. 3. Using beaker a. Mass in gh) of sample withour 250mL beaker tare b. Did taring the bealer tesutt in a larger, smalief, of equal sample mass determination than not tanng the beaker? Enter iaroor imathor, or equal liapt a awn to opan a tint Repod mass values for A3a in grams to the nearet 0.0019. Do not enter the unit 3. Uting beake a. Mass (n 0) of sample without 250 mt, beaker lare b. Did taing the be aser result in a larget, smafer, of equat sample mass detemination than not taring the beaken? Enter larget, smates, or equal 4. Compansors F you are unsure how to complete this section of the Aralysis, assietance is madatie in Hints Analysus 4a and Analyais 4b Scroti dewin to open a Hint. Eriter 1 for weighing papet, 2 tor aluminum weighing boat, and 3 for beaker. Einer lower number it two, or more, numben are correct. a. When the tare funcion on the botance is not ised 1. Which contanet choice resthed in the smalest sample mase determination? It Which contsiner choice reculted in the largest sample mass delermination? b. Whan the tare function on the batance is und I Which container choice resulted in the smatest saingle mass determination? is Which contalner choice resulted in the largect sample mats deternunation