Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Equation for Q4 is NaN3(s) = Na(s) + N2 1. A chemist wants to produce 7.50 g of barium sulfate with the following reaction. How
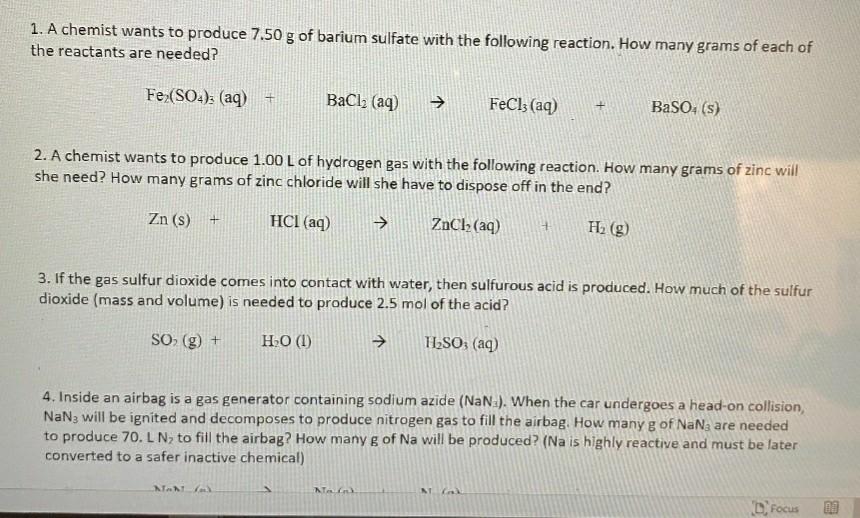
Equation for Q4 is NaN3(s) = Na(s) +N2
1. A chemist wants to produce 7.50 g of barium sulfate with the following reaction. How many grams of each of the reactants are needed? Fe (SO4)3 (aq) + BaCl2 (aq) FeCl3(aq) BaSO4(s) 2. A chemist wants to produce 1.00 L of hydrogen gas with the following reaction. How many grams of zinc will she need? How many grams of zinc chloride will she have to dispose off in the end? Zn (s) + HCl(aq) ZnCl2(aq) H2(g) 3. If the gas sulfur dioxide comes into contact with water, then sulfurous acid is produced. How much of the sulfur dioxide (mass and volume) is needed to produce 2.5 mol of the acid? SO(g) + HO (1) TLSO: (aq) 4. Inside an airbag is a gas generator containing sodium azide (NaN.). When the car undergoes a head-on collision, NaNg will be ignited and decomposes to produce nitrogen gas to fill the airbag. How many g of NaNa are needed to produce 70. L N, to fill the airbag? How many g of Na will be produced? (Na is highly reactive and must be later converted to a safer inactive chemical) No NG D. FocusStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


