Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Estimate the composition of the liquid and vapor phases when ethylene reacts with water to form ethanol at 200C and 34.5 bar, conditions which
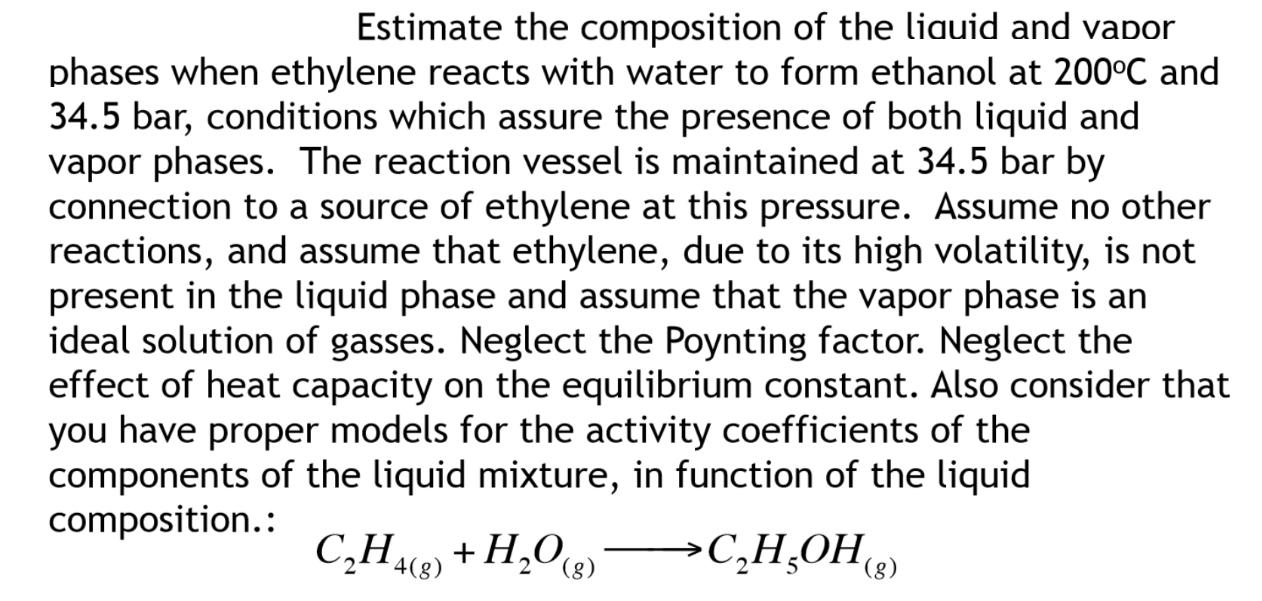
Estimate the composition of the liquid and vapor phases when ethylene reacts with water to form ethanol at 200C and 34.5 bar, conditions which assure the presence of both liquid and vapor phases. The reaction vessel is maintained at 34.5 bar by connection to a source of ethylene at this pressure. Assume no other reactions, and assume that ethylene, due to its high volatility, is not present in the liquid phase and assume that the vapor phase is an ideal solution of gasses. Neglect the Poynting factor. Neglect the effect of heat capacity on the equilibrium constant. Also consider that you have proper models for the activity coefficients of the components of the liquid mixture, in function of the liquid composition.: CH4(8) + HO(g) >CHOH(g)
Step by Step Solution
★★★★★
3.29 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION To estimate the composition of the liquid and vapor phases when ethylene reacts with water to form ethanol at 200C and 345 bar we can use the Gibbs free energy equation for a reaction G H TS ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


