Answered step by step
Verified Expert Solution
Question
1 Approved Answer
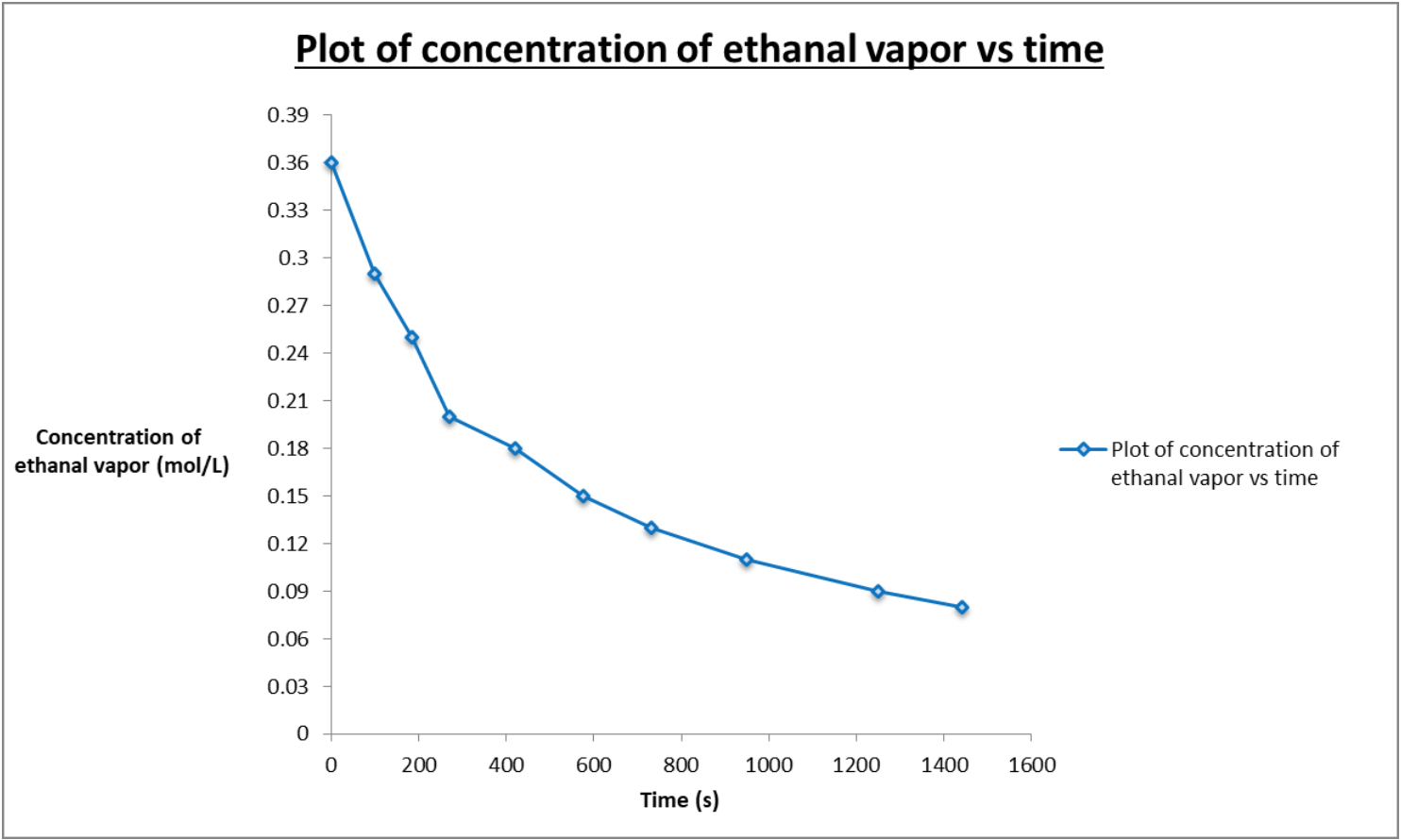
Ethanal vapour goes through thermal decomposition in a chemical reaction C2H4O(g) CH4(g) + CO(g) This is the graph: a) Look at the graph find the
Ethanal vapour goes through thermal decomposition in a chemical reaction C2H4O(g) CH4(g) + CO(g)
This is the graph:
a) Look at the graph find the instantaneous rate of decomposition of CHO when the concentration is 0.200 mol/L at 270 s. Place the tangent line on the curve. b) Determine the initial rate of reaction on the graph then place the tangent line on the curve.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


