Answered step by step
Verified Expert Solution
Question
1 Approved Answer
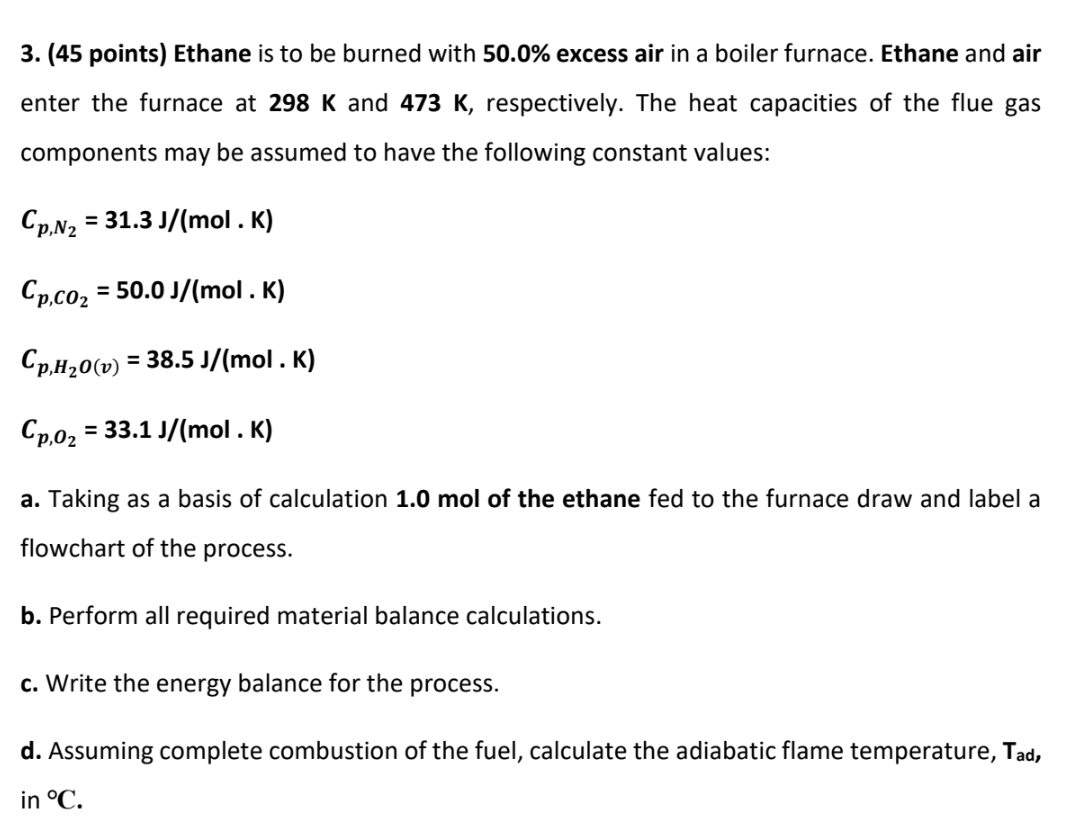
Ethane is to be burned with 5 0 . 0 % excess air in a boiler furnace. Ethane and air enter the furnace at 2
Ethane is to be burned with excess air in a boiler furnace. Ethane and air
enter the furnace at and respectively. The heat capacities of the flue gas
components may be assumed to have the following constant values:
a Taking as a basis of calculation mol of the ethane fed to the furnace draw and label a
flowchart of the process.
b Perform all required material balance calculations.
c Write the energy balance for the process.
d Assuming complete combustion of the fuel, calculate the adiabatic flame temperature,
in
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


