Answered step by step
Verified Expert Solution
Question
1 Approved Answer
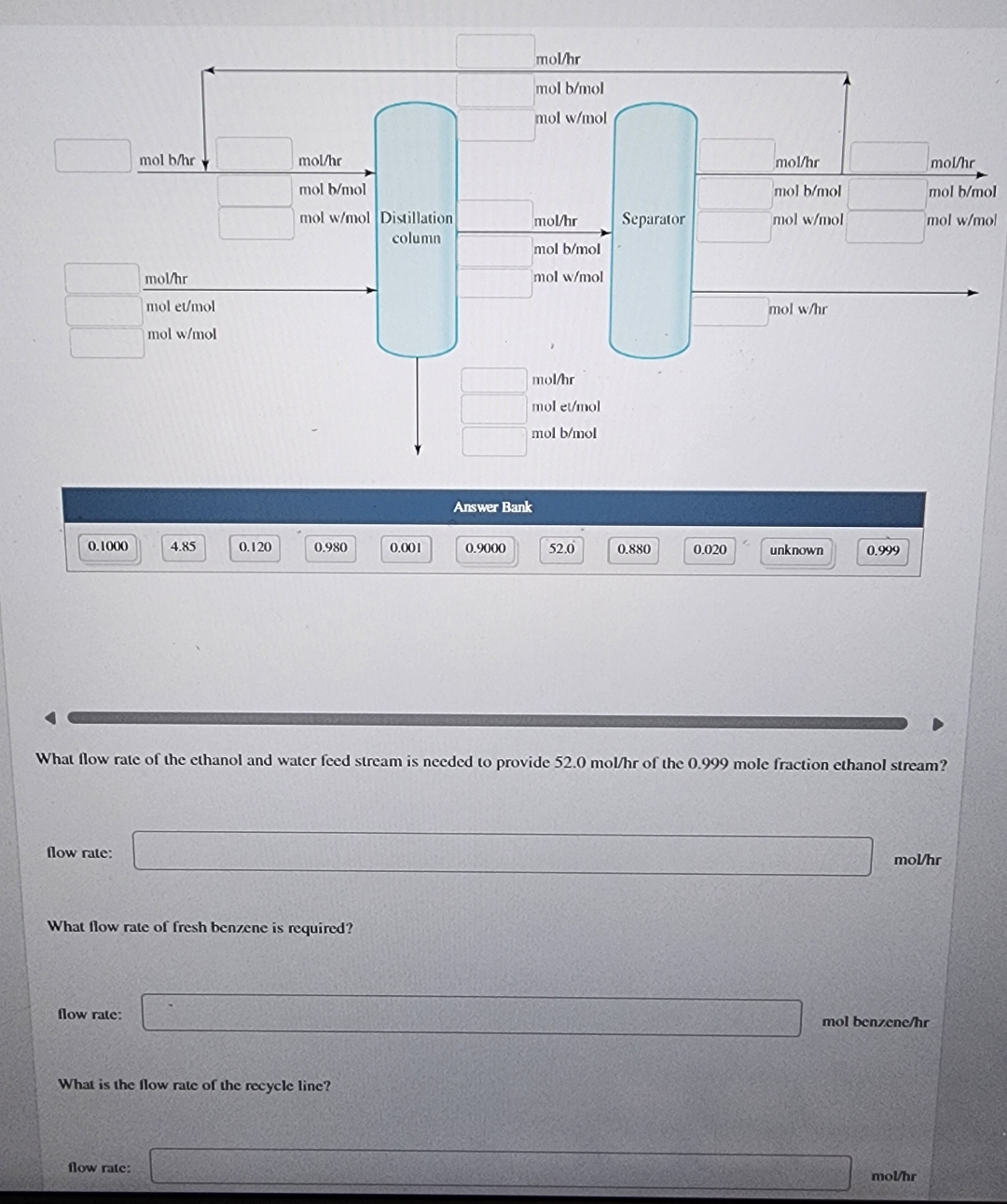
Ethanol is often used as a fuel additive, but to function in most engines, the ethanol must be very pure and contain little water. It
Ethanol is often used as a fuel additive, but to function in most engines, the ethanol must be very pure and contain little water. It is difficult to distill ethanol and water above mole percent ethanol because at this concentration, the vapor and the liquid phases of this mixture have the same composition. To get around this effect, benzene is often added to the mixture. The distillation column then separates the mixture into a very nearly pure ethanol stream and a mixture of benzene and water. The benzene may be separated from the water and then recycled to use again in the ethanolwater distillation.
A company wishes to distill a mole fraction ethanol mole fraction water solution. To aid in the distillation process, a mole fraction benzene mole fraction water mixture containing fresh benzene and a recovered benzene solution is added. The plant wishes to produce of a mole fraction ethanol mole fraction benzene mixture from a distillation column. The other product from the distillation is a water and benzene mixture. This mixture is sent to a separator which produces a stream of water, and a mole fraction benzene mole fraction water
Answer Bank
What flow rate of the ethanol and water feed stream is needed to provide of the mole fraction ethanol stream?
flow rate:
What flow rate of fresh benzene is required?
flow rate:
mol benzenehr
What is the flow rate of the recycle line?
flow rate:
molhr
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


