Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Ethyl acetate is used as a solvent for the extraction of caffeine from coffee beans in a membrane extraction unit. A key parameter that determines
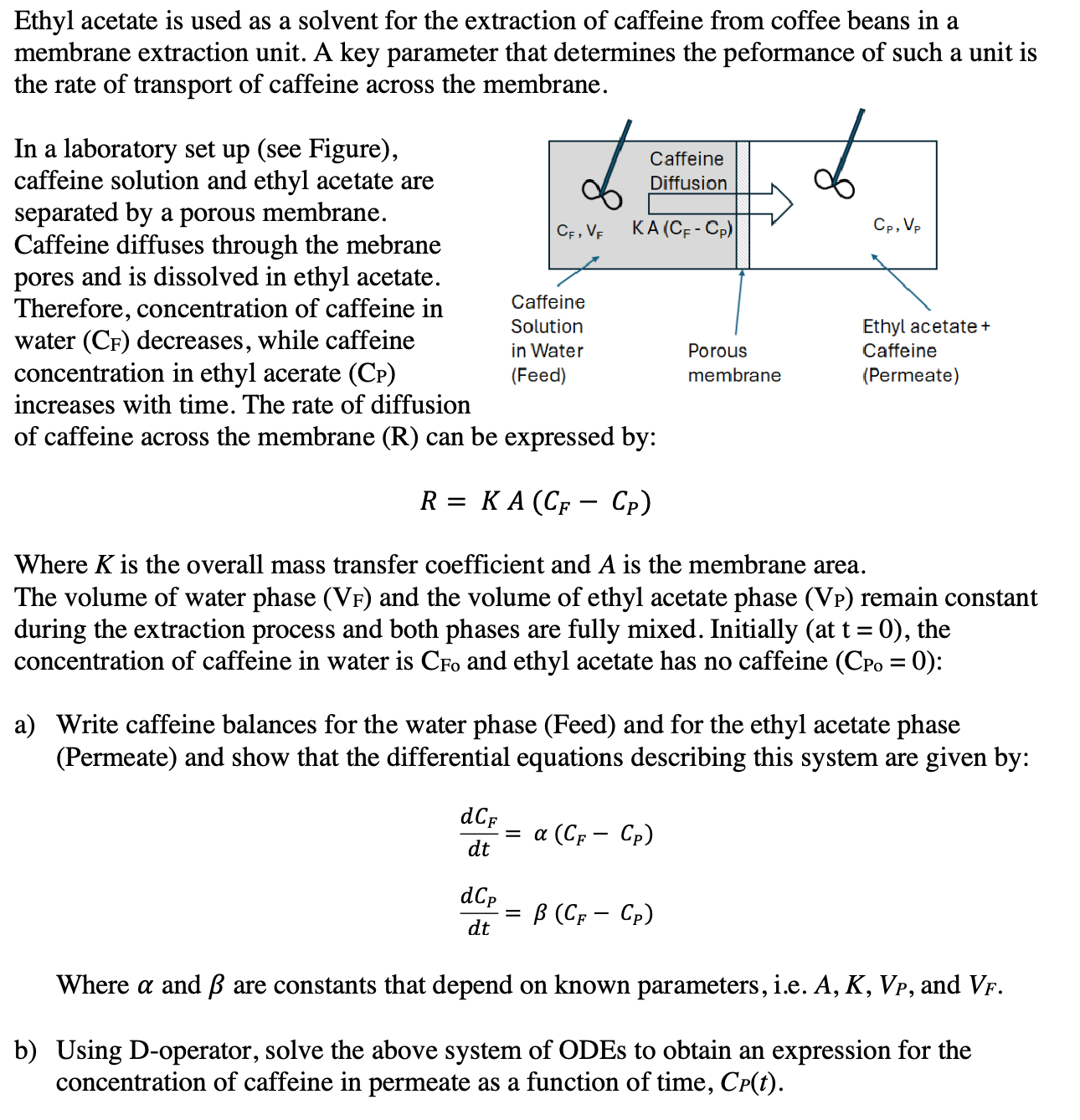
Ethyl acetate is used as a solvent for the extraction of caffeine from coffee beans in a membrane extraction unit. A key parameter that determines the peformance of such a unit is the rate of transport of caffeine across the membrane. In a laboratory set up see Figure
caffeine solution and ethyl acetate are separated by a porous membrane. Caffeine diffuses through the mebrane pores and is dissolved in ethyl acetate. Therefore, concentration of caffeine in water decreases, while caffeine concentration in ethyl acerate increases with time. The rate of diffusion of caffeine across the membrane can be expressed by:
Where is the overall mass transfer coefficient and is the membrane area. The volume of water phase and the volume of ethyl acetate phase remain constant during the extraction process and both phases are fully mixed. Initially the concentration of caffeine in water is and ethyl acetate has no caffeine :
a Write caffeine balances for the water phase Feed and for the ethyl acetate phase Permeate and show that the differential equations describing this system are given by:
Where and are constants that depend on known parameters, ie and
b Using Doperator, solve the above system of ODEs to obtain an expression for the concentration of caffeine in permeate as a function of time,
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


