Answered step by step
Verified Expert Solution
Question
1 Approved Answer
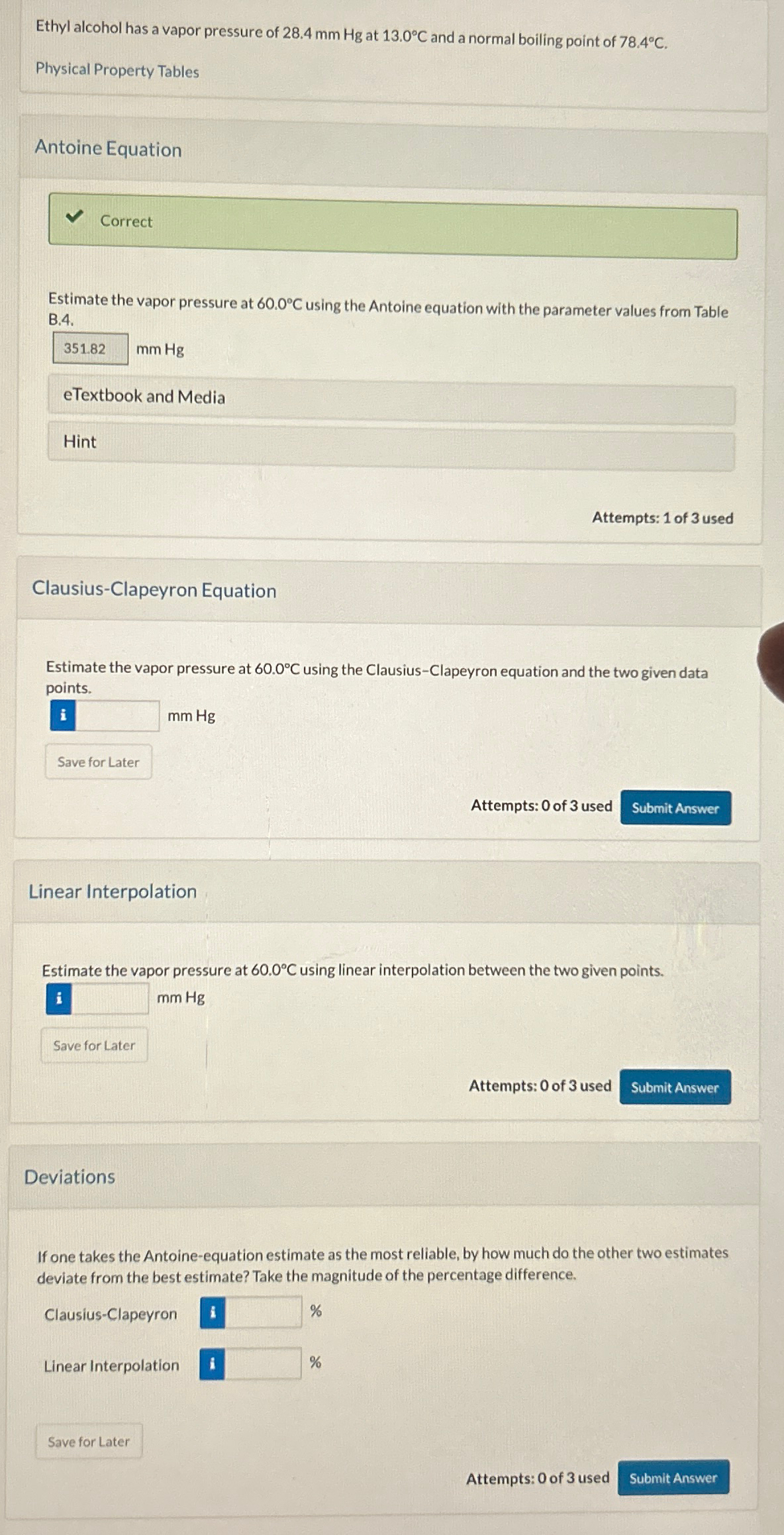
Ethyl alcohol has a vapor pressure of 2 8 . 4 m m H g at 1 3 . 0 C and a normal boiling
Ethyl alcohol has a vapor pressure of at and a normal boiling point of
Physical Property Tables
Antoine Equation
Correct
Estimate the vapor pressure at using the Antoine equation with the parameter values from Table B
eTextbook and Media
Hint
Attempts: of used
ClausiusClapeyron Equation
Estimate the vapor pressure at using the ClausiusClapeyron equation and the two given data points.
Attempts: of used
Linear Interpolation
Estimate the vapor pressure at using linear interpolation between the two given points.
Deviations
Attempts: of used
If one takes the Antoineequation estimate as the most reliable, by how much do the other two estimates deviate from the best estimate? Take the magnitude of the percentage difference.
ClausiusClapeyron
Linear Interpolation
Attempts: of used
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


