Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Ethylene and acetylene can be manufactured by the thermal cracking of the light ends of a refinery. The inlet gas to the cracker usually consists
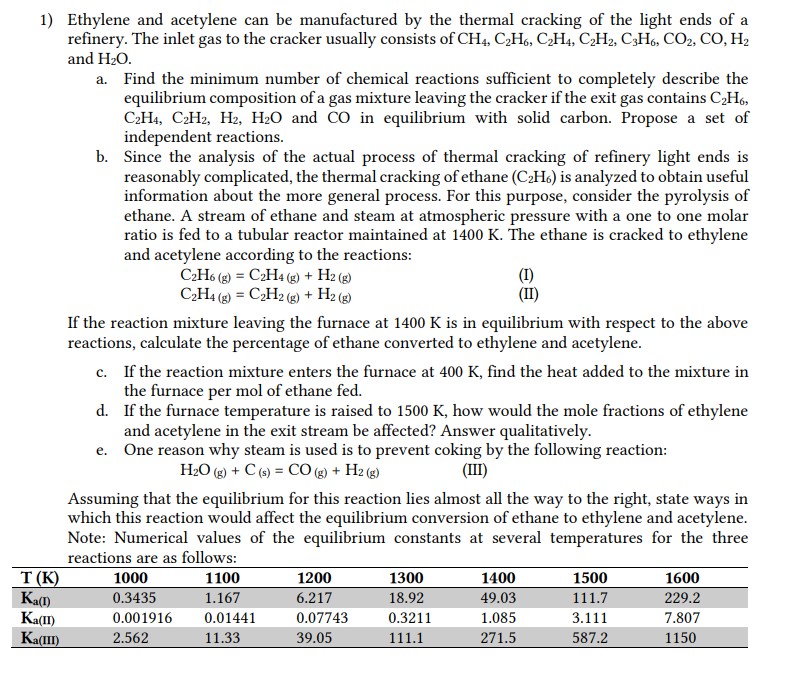 Ethylene and acetylene can be manufactured by the thermal cracking of the light ends of a refinery. The inlet gas to the cracker usually consists of CH4,C2H6,C2H4,C2H2,C3H6,CO2,CO,H2 and H2O. a. Find the minimum number of chemical reactions sufficient to completely describe the equilibrium composition of a gas mixture leaving the cracker if the exit gas contains C2H6, C2H4,C2H2,H2,H2O and CO in equilibrium with solid carbon. Propose a set of independent reactions. b. Since the analysis of the actual process of thermal cracking of refinery light ends is reasonably complicated, the thermal cracking of ethane (C2H6) is analyzed to obtain useful information about the more general process. For this purpose, consider the pyrolysis of ethane. A stream of ethane and steam at atmospheric pressure with a one to one molar ratio is fed to a tubular reactor maintained at 1400K. The ethane is cracked to ethylene and acetylene according to the reactions: C2H6(g)=C2H4g)+H2(g)C2H4(g)=C2H2(g)+H2(g) If the reaction mixture leaving the furnace at 1400K is in equilibrium with respect to the above reactions, calculate the percentage of ethane converted to ethylene and acetylene. c. If the reaction mixture enters the furnace at 400K, find the heat added to the mixture in the furnace per mol of ethane fed. d. If the furnace temperature is raised to 1500K, how would the mole fractions of ethylene and acetylene in the exit stream be affected? Answer qualitatively. e. One reason why steam is used is to prevent coking by the following reaction: H2O(g)+C(s)=CO(g)+H2(g) Assuming that the equilibrium for this reaction lies almost all the way to the right, state ways in which this reaction would affect the equilibrium conversion of ethane to ethylene and acetylene. Note: Numerical values of the equilibrium constants at several temperatures for the three reactions are as follows
Ethylene and acetylene can be manufactured by the thermal cracking of the light ends of a refinery. The inlet gas to the cracker usually consists of CH4,C2H6,C2H4,C2H2,C3H6,CO2,CO,H2 and H2O. a. Find the minimum number of chemical reactions sufficient to completely describe the equilibrium composition of a gas mixture leaving the cracker if the exit gas contains C2H6, C2H4,C2H2,H2,H2O and CO in equilibrium with solid carbon. Propose a set of independent reactions. b. Since the analysis of the actual process of thermal cracking of refinery light ends is reasonably complicated, the thermal cracking of ethane (C2H6) is analyzed to obtain useful information about the more general process. For this purpose, consider the pyrolysis of ethane. A stream of ethane and steam at atmospheric pressure with a one to one molar ratio is fed to a tubular reactor maintained at 1400K. The ethane is cracked to ethylene and acetylene according to the reactions: C2H6(g)=C2H4g)+H2(g)C2H4(g)=C2H2(g)+H2(g) If the reaction mixture leaving the furnace at 1400K is in equilibrium with respect to the above reactions, calculate the percentage of ethane converted to ethylene and acetylene. c. If the reaction mixture enters the furnace at 400K, find the heat added to the mixture in the furnace per mol of ethane fed. d. If the furnace temperature is raised to 1500K, how would the mole fractions of ethylene and acetylene in the exit stream be affected? Answer qualitatively. e. One reason why steam is used is to prevent coking by the following reaction: H2O(g)+C(s)=CO(g)+H2(g) Assuming that the equilibrium for this reaction lies almost all the way to the right, state ways in which this reaction would affect the equilibrium conversion of ethane to ethylene and acetylene. Note: Numerical values of the equilibrium constants at several temperatures for the three reactions are as follows Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


