Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Ethylene oxide ( C 2 H 4 O ) is used primarily as a sterilizing agent for medical equipment and supplies, and as a chemical
Ethylene oxide is used primarily as a sterilizing agent for medical equipment and supplies, and as a chemical intermediate in the production of ethylene glycol and other chemicals. It is produced by the catalytic oxidation of ethylene :
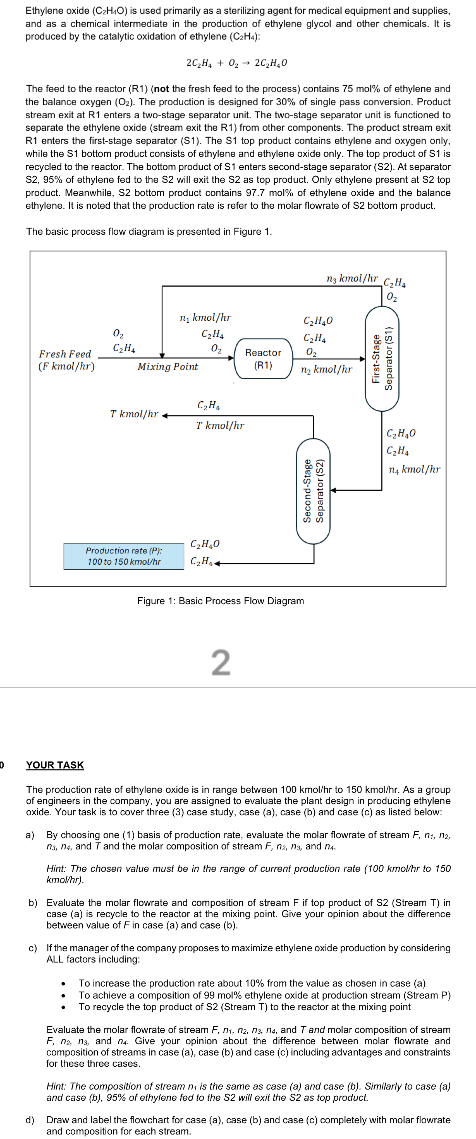
The feed to the reactor Rnot the fresh feed to the process contains mol of ethylene and the balance oxygen The production is designed for of single pass conversion. Product stream exit at R enters a twostage separator unit. The twostage separator unit is functioned to separate the ethylene oxide stream exit the R from other components. The product stream exit R enters the firststage separator S The S top product contains ethylene and oxygen only. while the bottom product consists of ethylene and ethylene oxide only. The top product of is recycled to the reactor. The bottom product of enters secondstage separator S At separator of ethylene fed to the will exit the as top product. Only ethylene present at top product. Meanwhile, S bottom product contains mol of ethylene oxide and the balance ethylene. It is noted that the production rate is refer to the molar flowrate of bottom product.
The basic process flow diagram is presented in Figure
YOUR TASK
The production rate of ethylene oxide is in range between kmo to kmo As a group of engineers in the company, you are assigned to evaluate the plant design in producing ethylene oxide. Your task is to cover three case study, case a case b and case c as listed below:
a By choosing one basis of production rate, evaluate the molar flowrate of stream and and the molar composition of stream and
Hint: The chosen value musf be in the range of currenf production rate kmo to kmolhry.
b Evaluate the molar flowrate and composition of stream if top product of Stream in case a is recycle to the reactor at the mixing point. Give your opinion about the difference between value of in case a and case b
c If the manager of the company proposes to maximize ethylene oxide production by considering ALL factors including:
To increase the production rate about from the value as chosen in case a
To achieve a composition of mol ethylene oxide at production stream Stream P
To recycle the top product of Stream T to the reactor at the mixing point
Evaluate the molar flowrate of stream and and molar composition of stream and Give your opinion about the difference between molar flowrate and composition of streams in case a case b and case c including advantages and constraints for these three cases.
Hint: The composition of stream is the same as case a and case b Similarly to case a and case b of ethylene fed to the will exit the as top product.
d Draw and label the flowchart for case a case b and case c completely with molar flawrate and composition for each stream.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


