Answered step by step
Verified Expert Solution
Question
1 Approved Answer
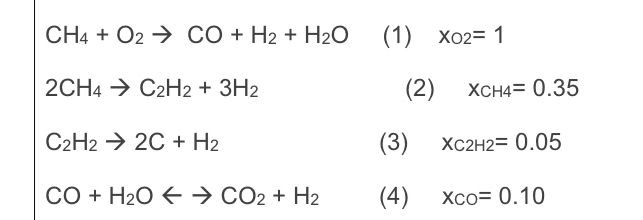
evaluate the following process used to produceacetylene from methane. The following reactions takes place during the process. The feed flow of methane is 2 0
evaluate the following process used to produceacetylene from methane. The following reactions takes place during the process. The feed flow of methane is kgmoleh pure andoxygen enough is used to have full conversion of oxygen and enough heatto trigger the desired pyrolysis of methane to produce acetylen.....Calculate the extent of the reaction.
Using HYSYS design the process considering the following aspects:
The fluid package is Extended NRTL
The acetylene production is achieved by pyrolysis of methane with a
constant feed of methane and oxygen at oC and constant
pressure bar.
The reaction is trigger by the heat generated by the exothermic
combustion reaction at constant pressure.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


