Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Everything but the final question has been solved, the correct answers are shown in bold. Please use the information from previous answers to solve the
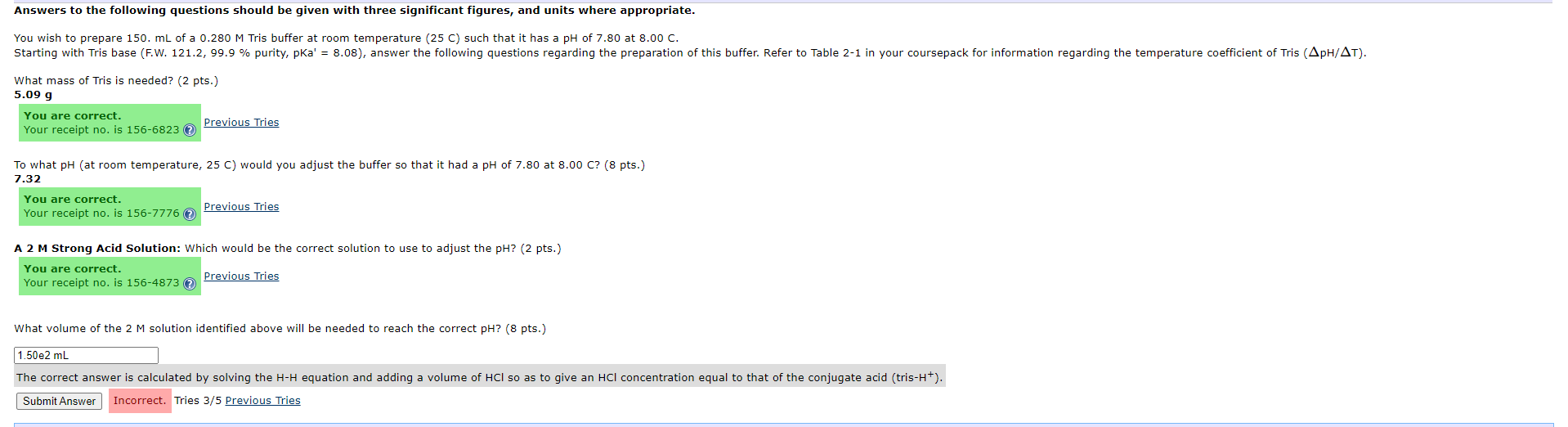
Everything but the final question has been solved, the correct answers are shown in bold. Please use the information from previous answers to solve the last question.
Answers to the following questions should be given with three significant figures, and units where appropriate. You wish to prepare 150. mL of a 0.280M Tris buffer at room temperature ( 25C ) such that it has a pH of 7.80 at 8.00C. What mass of Tris is needed? (2 pts.) Previous Tries To what pH (at room temperature, 25C ) would you adjust the buffer so that it had a pH of 7.80 at 8.00C ? ( 8pts ) 7.32 Previous Tries A 2 M Strong Acid Solution: Which would be the correct solution to use to adjust the pH? (2 pts.) What volume of the 2M solution identified above will be needed to reach the correct pH? ( 8 pts.) The correct answer is calculated by solving the HH equation and adding a volume of HCl so as to give an HCl concentration equal to that of the conjugate acid (tris- H+). Tries 3/5 Previous TriesStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


