Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Examine the conditions under which a liquid Fe-Mn alloy can be in equilibrium with an FeO-MnO liquid solution in an atmosphere containing oxygen at
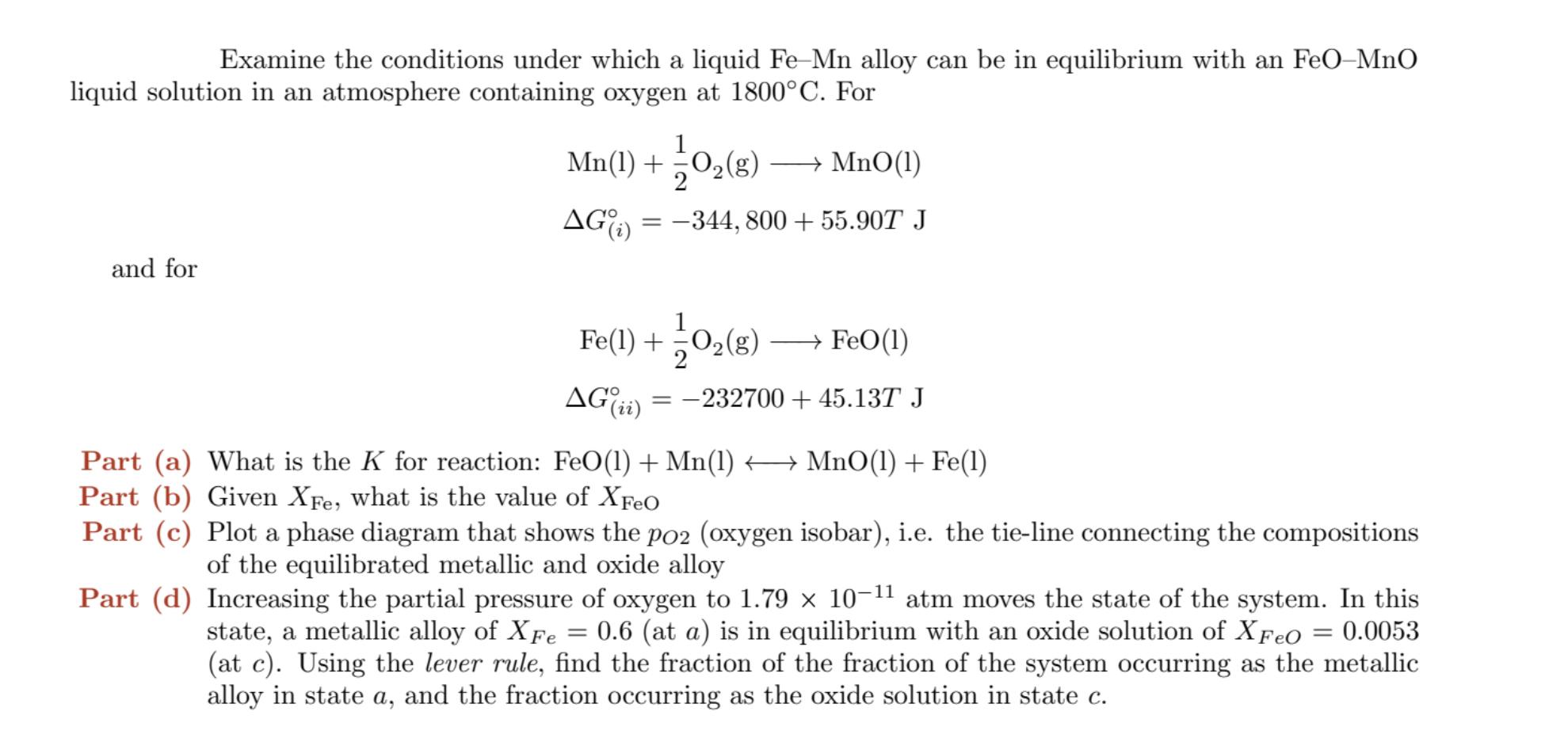
Examine the conditions under which a liquid Fe-Mn alloy can be in equilibrium with an FeO-MnO liquid solution in an atmosphere containing oxygen at 1800C. For and for Mn(1) + AG (i) = O(g) MnO(1) -344, 800 + 55.90T J Fe(1) + O(g) FeO(1) AG(ii) = 232700 + 45.13T J Part (a) What is the K for reaction: Part (b) Given XFe, what is the value of XFeO Part (c) Plot a phase diagram that shows the po2 (oxygen isobar), i.e. the tie-line connecting the compositions of the equilibrated metallic and oxide alloy FeO(1) + Mn(1) MnO(1) + Fe(1) Part (d) Increasing the partial pressure of oxygen to 1.79 10-11 atm moves the state of the system. In this state, a metallic alloy of XFe = 0.6 (at a) is in equilibrium with an oxide solution of XFeO = 0.0053 (at c). Using the lever rule, find the fraction of the fraction of the system occurring as the metallic alloy in state a, and the fraction occurring as the oxide solution in state c.
Step by Step Solution
★★★★★
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Answer Hance solved it Total anable 6 Xfe Xmn Part ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


