Question
A piston-cylinder device initially contains 0.4 m3 of air at 100 kPa and 80C. The air is now compressed to 0.1 m in such
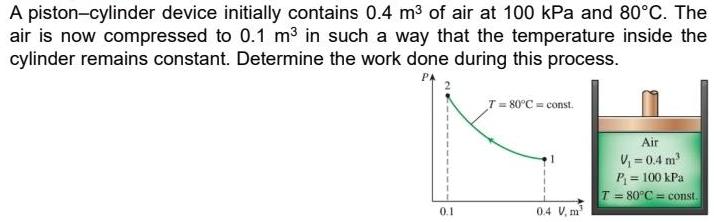
A piston-cylinder device initially contains 0.4 m3 of air at 100 kPa and 80C. The air is now compressed to 0.1 m in such a way that the temperature inside the cylinder remains constant. Determine the work done during this process. T= 80C = const. Air , =0.4 m P = 100 kPa T = 80C = const. 0.1 0.4 V, m
Step by Step Solution
3.39 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



