Question
Reported values for the virial coefficients of isopropanol vapor at 200C are: B = -388 cm3/mol C = -26,000 cm/mol? Calculate v and Z
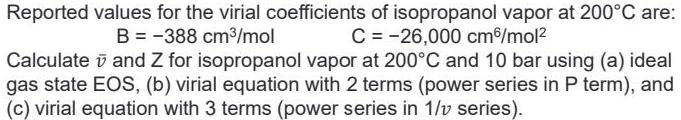
Reported values for the virial coefficients of isopropanol vapor at 200C are: B = -388 cm3/mol C = -26,000 cm/mol? Calculate v and Z for isopropanol vapor at 200C and 10 bar using (a) ideal gas state EOS, (b) virial equation with 2 terms (power series in P term), and (c) virial equation with 3 terms (power series in 1/v series).
Step by Step Solution
3.39 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Chemical Engineering Thermodynamics
Authors: J. M. Smith, H. C. Van Ness, M. M. Abbott
7th edition
71247084, 978-0071247085
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



