Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Example 30.1 Calculating the maximum available work When 1.000 mol CH,0, (glucose) is oxidized to carbon dioxide and water at 25C according to the equation
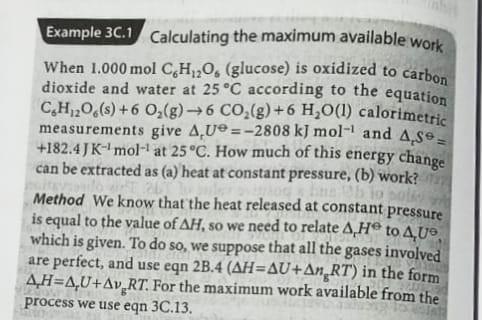
Example 30.1 Calculating the maximum available work When 1.000 mol CH,0, (glucose) is oxidized to carbon dioxide and water at 25C according to the equation CH,20 (s) +6 O2(g) 6 CO2(g) +6 H,O(1) calorimetric measurements give 4 U =-2808 kJ mol-l and 4,39 +182.45 K-mol-1 at 25C. How much of this energy change can be extracted as (a) heat at constant pressure, (b) work? Sole Method We know that the heat released at constant pressure is equal to the value of AH, so we need to relate A, H to 4,0 which is given. To do so, we suppose that all the gases involved are perfect, and use eqn 2B.4 (AH=AU+An RT) in the form 4H=4U+Av,RT. For the maximum work available from the process we use eqn 3C.13
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


