Question
A rigid tank is divided by a partition into 2 equal parts. Initially, one side of the tank has 5 kg of water at
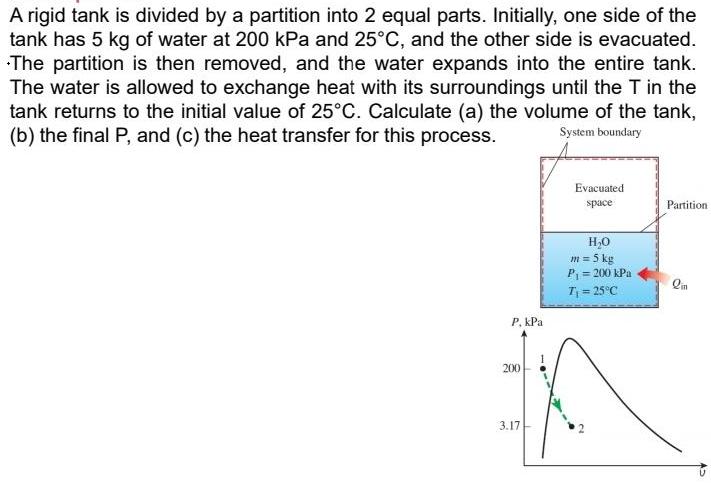
A rigid tank is divided by a partition into 2 equal parts. Initially, one side of the tank has 5 kg of water at 200 kPa and 25C, and the other side is evacuated. The partition is then removed, and the water expands into the entire tank. The water is allowed to exchange heat with its surroundings until the T in the tank returns to the initial value of 25C. Calculate (a) the volume of the tank, (b) the final P, and (c) the heat transfer for this process. System boundary Evacuated space Partition H,0 m = 5 kg P = 200 kPa T = 25C P. kPa 200 3.17
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



