Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Example 34.1 Calculating the entropy change for the isothermal expansion of a perfect gas Calculate the entropy change of a sample of perfect gas when
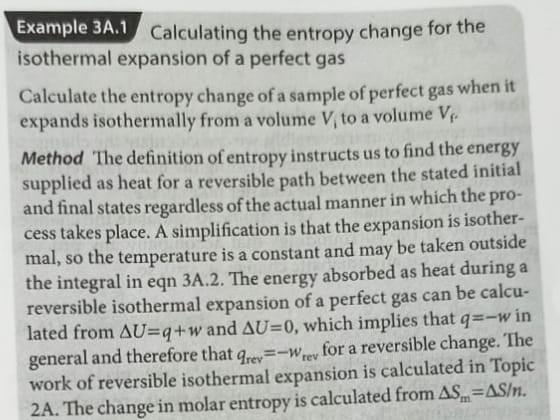
Example 34.1 Calculating the entropy change for the isothermal expansion of a perfect gas Calculate the entropy change of a sample of perfect gas when it expands isothermally from a volume V, to a volume V Method "The definition of entropy instructs us to find the energy supplied as heat for a reversible path between the stated initial and final states regardless of the actual manner in which the pro- cess takes place. A simplification is that the expansion is isother- mal, so the temperature is a constant and may be taken outside the integral in eqn 3A.2. The energy absorbed as heat during a reversible isothermal expansion of a perfect gas can be calcu- lated from AU=q+w and AU=0, which implies that q=-w in general and therefore that grev=-Wrey for a reversible change. The work of reversible isothermal expansion is calculated in Topic 2A. The change in molar entropy is calculated from AS.=AS
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


