Answered step by step
Verified Expert Solution
Question
1 Approved Answer
EXAMPLE 4 . 1 0 - Removal of C u S O 4 from a Pollution Control Residue. A pollution - control residue containing copper
EXAMPLE Removal of from a Pollution Control Residue.
A pollutioncontrol residue containing copper oxide was leached with sulfuric acid to produce a slurry having a pulp density of The in the solution phase of the slurry is The slurry is washed countercurrently in three stages with water. The slurry moving between stages and discharge has the same volume fraction liquid as the entering slurry. The volumetric flow of liquid moving countercurrently to the slurry is the same as the volume of wash water. How much wash water should be used such that the discharge slurry contains of the incoming amount of copper? The slurry solids have a density of
Data. The approximate specific gravity of solutions is:
solution
Solution. Heretofore, we used a mass basis for material entering and traversing the system, but the sometimes the flow between mixersettler devices is specified on a volume basis. This occurs when a pump is set for a certain volumetric flow. The assumption of conservation of volume is
Chapter Fundamentals of Material Balances in NonReacting Systems
good when the solution is dilute, but in this example, the incoming solution is not dilute, having a density of Even though a constantvolume flow is specified between devices, volume is not necessarily conserved, so at some point, at least one stream volume must be left unspecified. In other words, it is not possible to specify arbitrarily the volume of every stream of a process.
For streams where the volume is specified, the mass balance equations require a volumetomass conversion. Please review Example for conversion arithmetic between density, volume, and mass of a slurry. Since no basis was specified, a volume basis of input slurry is chosen, which has a mass of
The first step is to calculate the mass of each phase in the feed and discharge slurry. For the feed slurry:
Mass balance: mass of liquid mass of solid
Volume balance: volume of liquid volume of solid
Recognizing that the volume is equal to massdensity the above two equations can be solved for the mass and volume of each phase of the feed slurry.
tableFeed slurry,mass, volume, Lmass fractionsolid solidliquid CuSO
Here the question and here the answer Solve using Mass balances
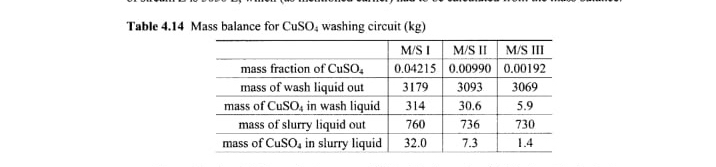
Table Mass balance for washing circuit
table I, II IIImass fraction of mass of wash liquid out,mass of in wash liquid,mass of slurry liquid out,mass of in slurry liquid,
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


