Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Exercise 1 0 . Sucrose ( C 1 2 H 2 2 O 1 1 ) undergoes hydrolysis to form fructose ( C 6 H
Exercise Sucrose undergoes hydrolysis to form fructose and glucose :
This reaction is important in the candy industry. First, fructose is sweeter than sucrose. In addition, a mixture of fructose and glucose will not crystallize. This makes the sweets elastic.
a Based on the following data, determine the order of the reaction
b How long does it take to hydrolyse sucrose?
min
first order
c Explain why the speed equation does not contain while water is a reagent.
water does no make sense to hue a concontration
Is doen't detect the rute
tableTime min
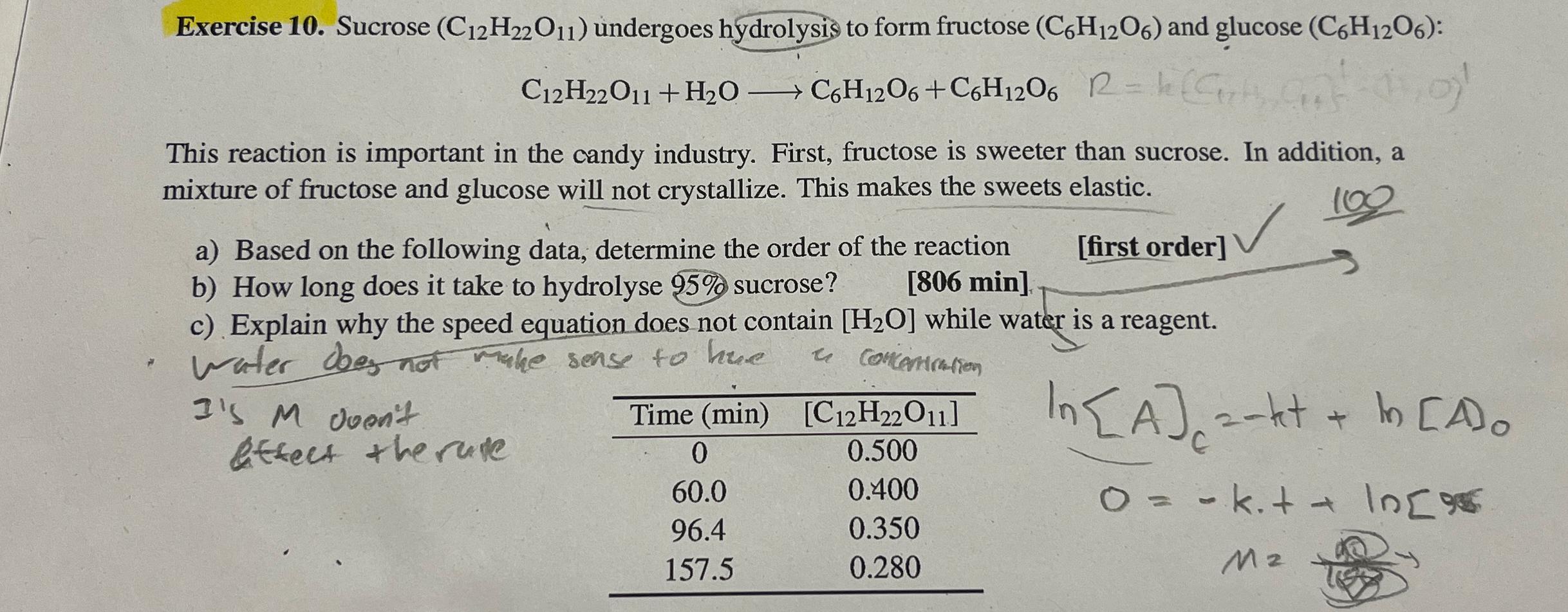
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


