Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The atmosphere of Mars is mostly CO2 (molar mass 44.0g/mol) under a pressure of 650 Pa, which we shall assume remains constant. In many
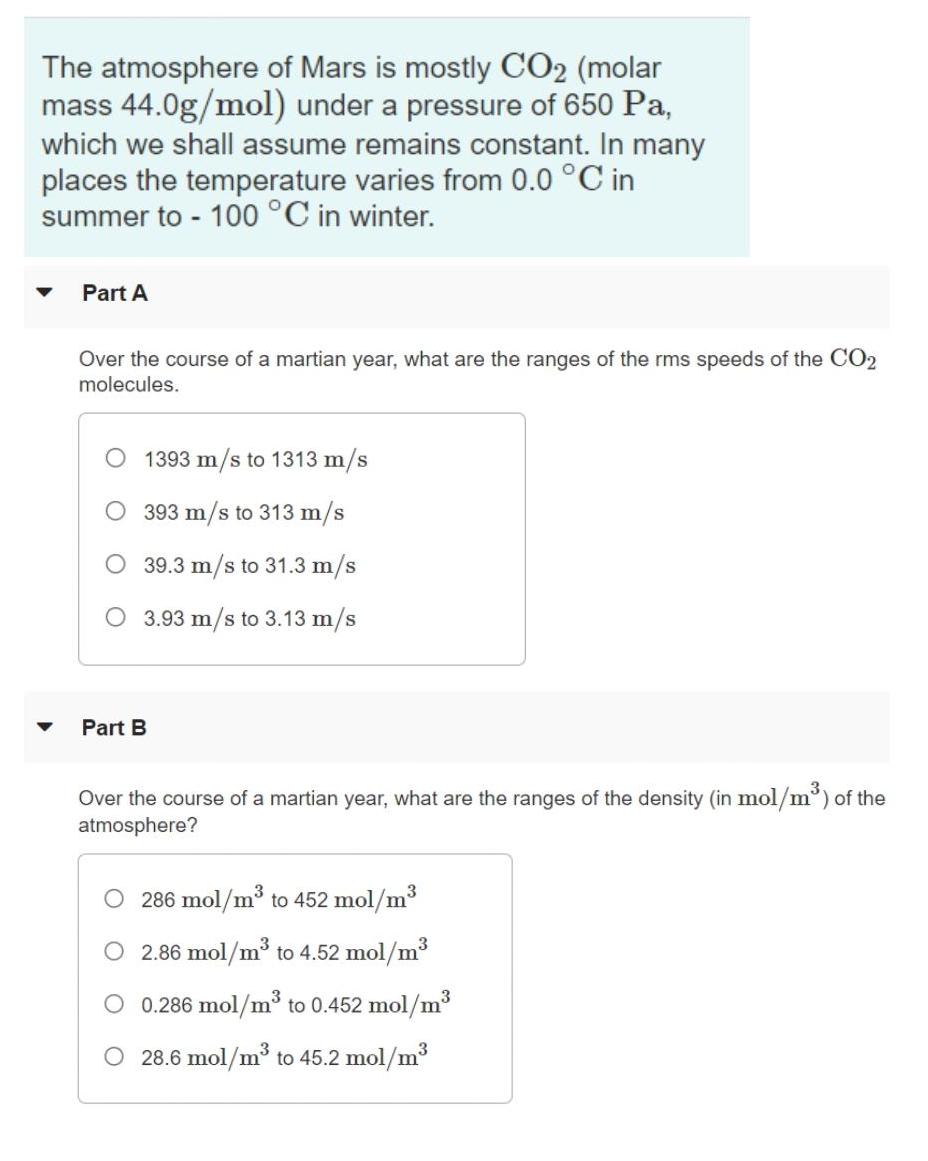
The atmosphere of Mars is mostly CO2 (molar mass 44.0g/mol) under a pressure of 650 Pa, which we shall assume remains constant. In many places the temperature varies from 0.0 C in summer to - 100 C in winter. Part A Over the course of a martian year, what are the ranges of the rms speeds of the CO2 molecules. O 1393 m/s to 1313 m/s 393 m/s to 313 m/s 39.3 m/s to 31.3 m/s 3.93 m/s to 3.13 m/s Part B Over the course of a martian year, what are the ranges of the density (in mol/m) of the atmosphere? O 286 mol/m to 452 mol/m3 O 2.86 mol/m to 4.52 mol/m3 0.286 mol/m to 0.452 mol/m3 28.6 mol/m to 45.2 mol/m
Step by Step Solution
★★★★★
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
608c00a37c77c_208107.pdf
180 KBs PDF File
608c00a37c77c_208107.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


