Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Exercise #2: Vapor-Liquid Equilibria (VLE) data for the ethanol (1) - benzene (2) system at 40C are presented below: Ihe vapor pressures of the pure
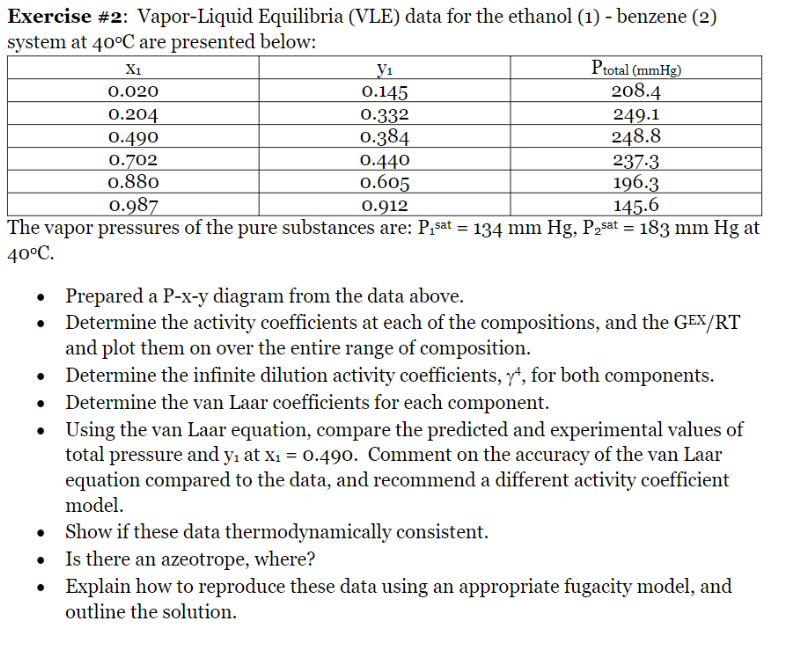 Exercise \#2: Vapor-Liquid Equilibria (VLE) data for the ethanol (1) - benzene (2) system at 40C are presented below: Ihe vapor pressures of the pure substances are: Y1sat=134mmHg,P2sat=183mmHg at 40C. - Prepared a P-x-y diagram from the data above. - Determine the activity coefficients at each of the compositions, and the GEX/RT and plot them on over the entire range of composition. - Determine the infinite dilution activity coefficients, 4, for both components. - Determine the van Laar coefficients for each component. - Using the van Laar equation, compare the predicted and experimental values of total pressure and y1 at x1=0.490. Comment on the accuracy of the van Laar equation compared to the data, and recommend a different activity coefficient model. - Show if these data thermodynamically consistent. - Is there an azeotrope, where? - Explain how to reproduce these data using an appropriate fugacity model, and outline the solution. Exercise \#2: Vapor-Liquid Equilibria (VLE) data for the ethanol (1) - benzene (2) system at 40C are presented below: Ihe vapor pressures of the pure substances are: Y1sat=134mmHg,P2sat=183mmHg at 40C. - Prepared a P-x-y diagram from the data above. - Determine the activity coefficients at each of the compositions, and the GEX/RT and plot them on over the entire range of composition. - Determine the infinite dilution activity coefficients, 4, for both components. - Determine the van Laar coefficients for each component. - Using the van Laar equation, compare the predicted and experimental values of total pressure and y1 at x1=0.490. Comment on the accuracy of the van Laar equation compared to the data, and recommend a different activity coefficient model. - Show if these data thermodynamically consistent. - Is there an azeotrope, where? - Explain how to reproduce these data using an appropriate fugacity model, and outline the solution
Exercise \#2: Vapor-Liquid Equilibria (VLE) data for the ethanol (1) - benzene (2) system at 40C are presented below: Ihe vapor pressures of the pure substances are: Y1sat=134mmHg,P2sat=183mmHg at 40C. - Prepared a P-x-y diagram from the data above. - Determine the activity coefficients at each of the compositions, and the GEX/RT and plot them on over the entire range of composition. - Determine the infinite dilution activity coefficients, 4, for both components. - Determine the van Laar coefficients for each component. - Using the van Laar equation, compare the predicted and experimental values of total pressure and y1 at x1=0.490. Comment on the accuracy of the van Laar equation compared to the data, and recommend a different activity coefficient model. - Show if these data thermodynamically consistent. - Is there an azeotrope, where? - Explain how to reproduce these data using an appropriate fugacity model, and outline the solution. Exercise \#2: Vapor-Liquid Equilibria (VLE) data for the ethanol (1) - benzene (2) system at 40C are presented below: Ihe vapor pressures of the pure substances are: Y1sat=134mmHg,P2sat=183mmHg at 40C. - Prepared a P-x-y diagram from the data above. - Determine the activity coefficients at each of the compositions, and the GEX/RT and plot them on over the entire range of composition. - Determine the infinite dilution activity coefficients, 4, for both components. - Determine the van Laar coefficients for each component. - Using the van Laar equation, compare the predicted and experimental values of total pressure and y1 at x1=0.490. Comment on the accuracy of the van Laar equation compared to the data, and recommend a different activity coefficient model. - Show if these data thermodynamically consistent. - Is there an azeotrope, where? - Explain how to reproduce these data using an appropriate fugacity model, and outline the solution Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


