Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the set of reversible, first-order reactions k k k3 A=B=C=D=E k-1 k-2 k-3 k-4 taking place in a well-mixed, batch reactor. The reactions
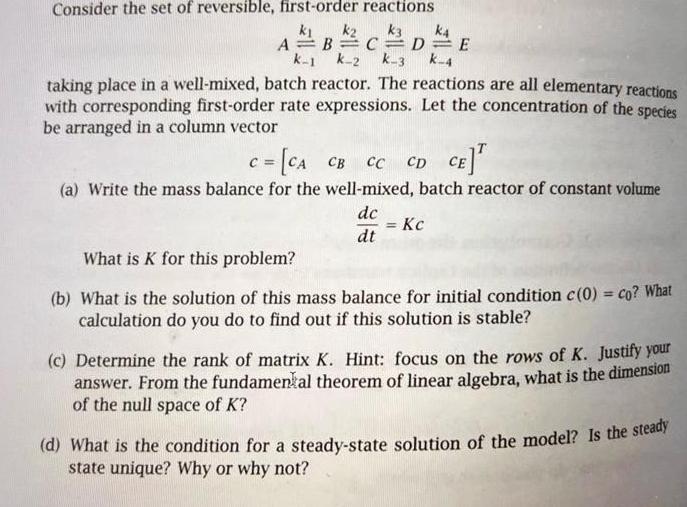
Consider the set of reversible, first-order reactions k k k3 A=B=C=D=E k-1 k-2 k-3 k-4 taking place in a well-mixed, batch reactor. The reactions are all elementary reactions with corresponding first-order rate expressions. Let the concentration of the species be arranged in a column vector c = [CA CB C CC CD CE] (a) Write the mass balance for the well-mixed, batch reactor of constant volume dc dt k4 = Kc What is K for this problem? (b) What is the solution of this mass balance for initial condition c(0) = co? What calculation do you do to find out if this solution is stable? (c) Determine the rank of matrix K. Hint: focus on the rows of K. Justify your answer. From the fundamental theorem of linear algebra, what is the dimension of the null space of K? (d) What is the condition for a steady-state solution of the model? Is the steady state unique? Why or why not?
Step by Step Solution
★★★★★
3.51 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Answer The reactions can be represented as follows 1Ak1BForward reaction 2Bk1AReverse reaction 3Bk2CForward reaction 4Ck2BReverse reaction 5Ck3DForwar...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


