Answered step by step
Verified Expert Solution
Question
1 Approved Answer
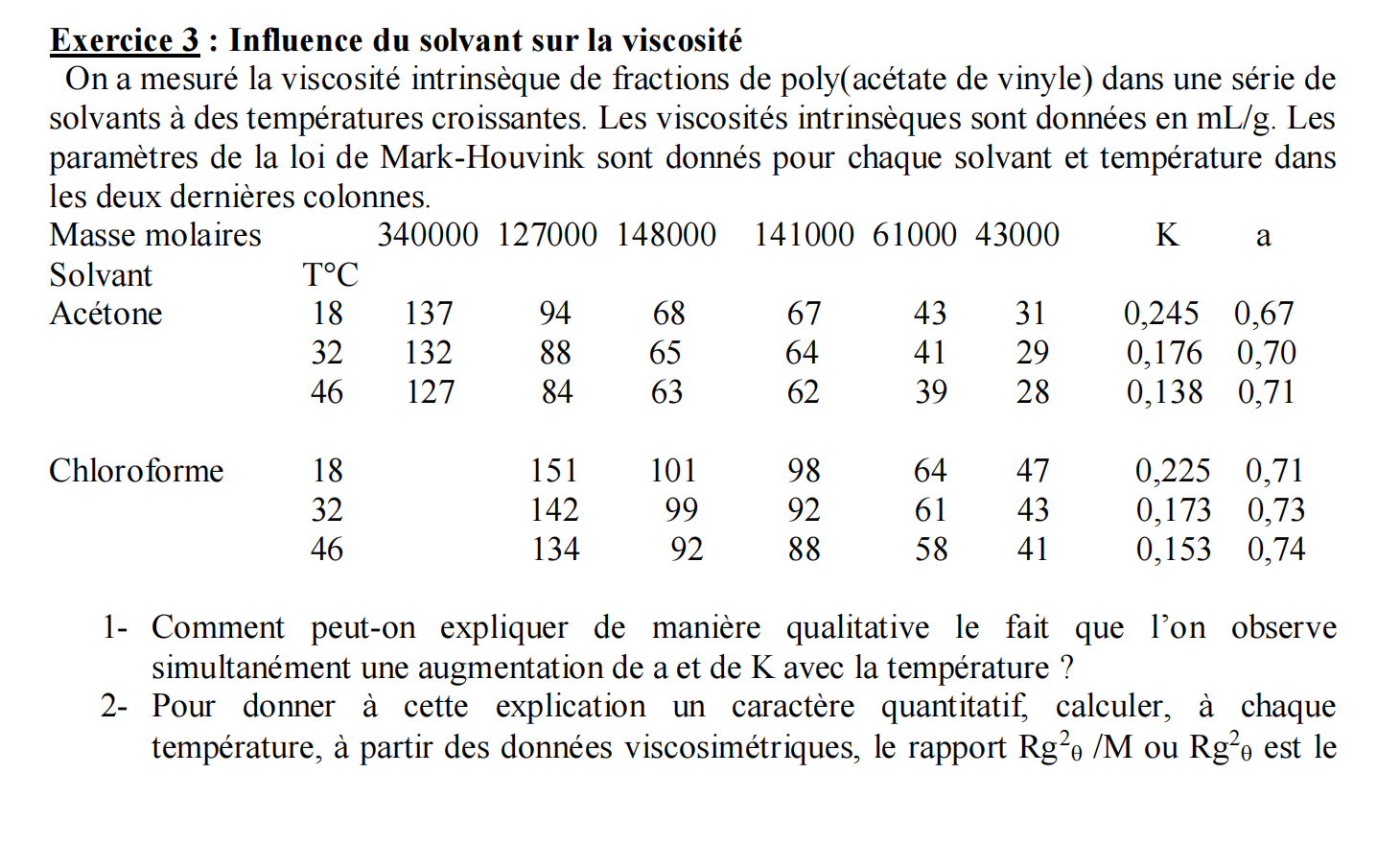
Exercise 3 : Influence of the solvent on viscosity The intrinsic viscosity of poly ( vinyl acetate ) fractions was measured in a series of
Exercise : Influence of the solvent on viscosity
The intrinsic viscosity of polyvinyl acetate fractions was measured in a series of
solvents at increasing temperatures. The intrinsic viscosities are given in the parameters of the MarkHouvink law are given for each solvent and temperature in the last two columns.
How can we explain qualitatively the fact that we observe
simultaneously an increase in a and K with temperature?
To give this explanation a quantitative character, calculate, at each
temperature, from viscosimetric data, the ratio or theta We will take the FloxFlory law. Deduce the length of the statistical link.
Compare the undisturbed dimensions obtained:
has. At the same temperature in two solvents. Comment
b At different temperatures in the same solvent. Is the observed variation compatible with that expected for a chain with a valence angle and symmetrical hindered rotation potential on the valence cone.
Could a variation of inverse sign also be observed? how to interpret it
Based on the data obtained for the largest molar mass in
acetone, calculate the variation of the expansion coefficient a of the chain with the
temperature. How can we interpret it
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


