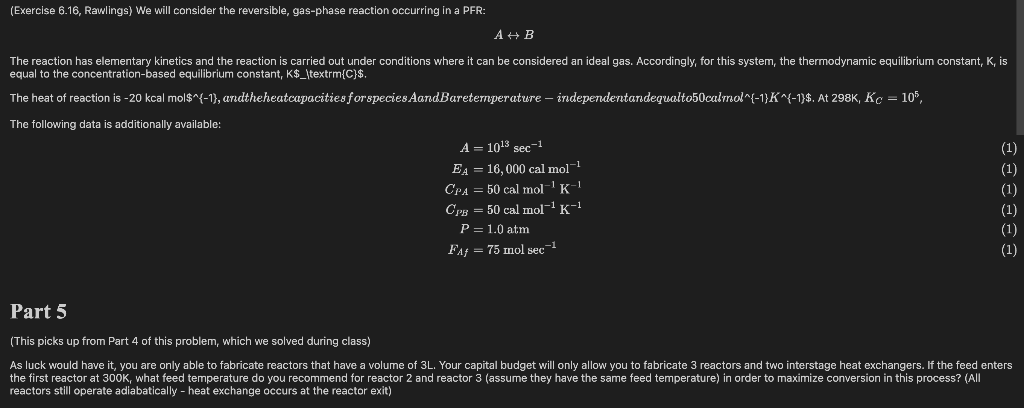

(Exercise 6.16, Rawlings) We will consider the reversible, gas-phase reaction occurring in a PFR: The reaction has elementary kinetics and the reaction is carried out under conditions where it can be considered an ideal gas. Accordingly, for this system, the thermodynamic equilibrium constant, K, is equal to the concentration-based equilibrium constant, K$_\textrm{C}$. The heat of reaction is -20 kcal mol$^{-1}, andtheheatcapacities for species AandBaretemperature independentardequalto50calmol^{-1}K^{-1}$. At 298K, Kc = 105, The following data is additionally available: A = 1013 sec-1 EA = 16,000 cal mol-? CPA = 50 cal mol-'K-1 Cpg = 50 cal mol-K-1 P=1.0 atm F f = 75 mol sec-1 (1) (1) (1) (1) (1) (1) Part 5 (This picks up from Part 4 of this problem, which we solved during class) As luck would have it, you are only able to fabricate reactors that have a volume of 3L. Your capital budget will only allow you to fabricate 3 reactors and two interstage heat exchangers. If the feed enters the first reactor at 300K, what feed temperature do you recommend for reactor 2 and reactor 3 (assume they have the same feed temperature) in order to maximize conversion in this process? (All reactors still operate adiabatically - heat exchange occurs at the reactor exit) Part 6 Assume that the annualized capital cost of constructing a single PFR scales with reactor volume according to Cost = 50,000 V. Here, V is in units of L and Cost is in \$ per year. You are currently able to sell the product, B, for ($1.75 mol$^{-1}$, and you expect this price to be relatively stable for the lifetime of your project. You purchase species A at ($0.35 mol$^{-1}$. Note that your maintenance costs will increase with the number of reactors; it costs you 5 x 108 \$ per year to maintain a single reactor; for this simple exercise, assume that maintenance costs double if you add a second reactor, triple if you add a third reactor, etc. You may neglect the cost of purchasing heat exchangers and pumping cooling water through the heat exchangers, and you may compute an annual profit as: Profit = Annual Revenue Annualized Cost - Annual Raw Material Cost Annual Maintenance Cost You supply species A at a rate of 75 mol sec$^{-1}$ into the first reactor. As above, you carry out this process in a sequence of PFRs with interstage coolers, and your goal is still to maximize profit given all of the revenue and cost streams specified above. In this case, we'll consider a more flexible situation where we have control over the number of reactors, the volume of each reactor, and the feed temperature to each reactor. Specifically, with the goal of maximizing profit for this system, answer: 1. How many PFRs in series should you use 2. What is the volume of each reactor (assume all reactors have equal volume, for simplicity) 3. What feed temperature should the heat exchangers cool the process stream to prior to entering the reactors. For simpliity, assume that the feed to each reactor - including the first reactor - has the exact same feed temperature








