Question
Exp. 2 Fractional Distillation Introduction: The purpose of this experiment is to learn how to differentiate, set-up, and use a fractional distillation apparatus to efficiently
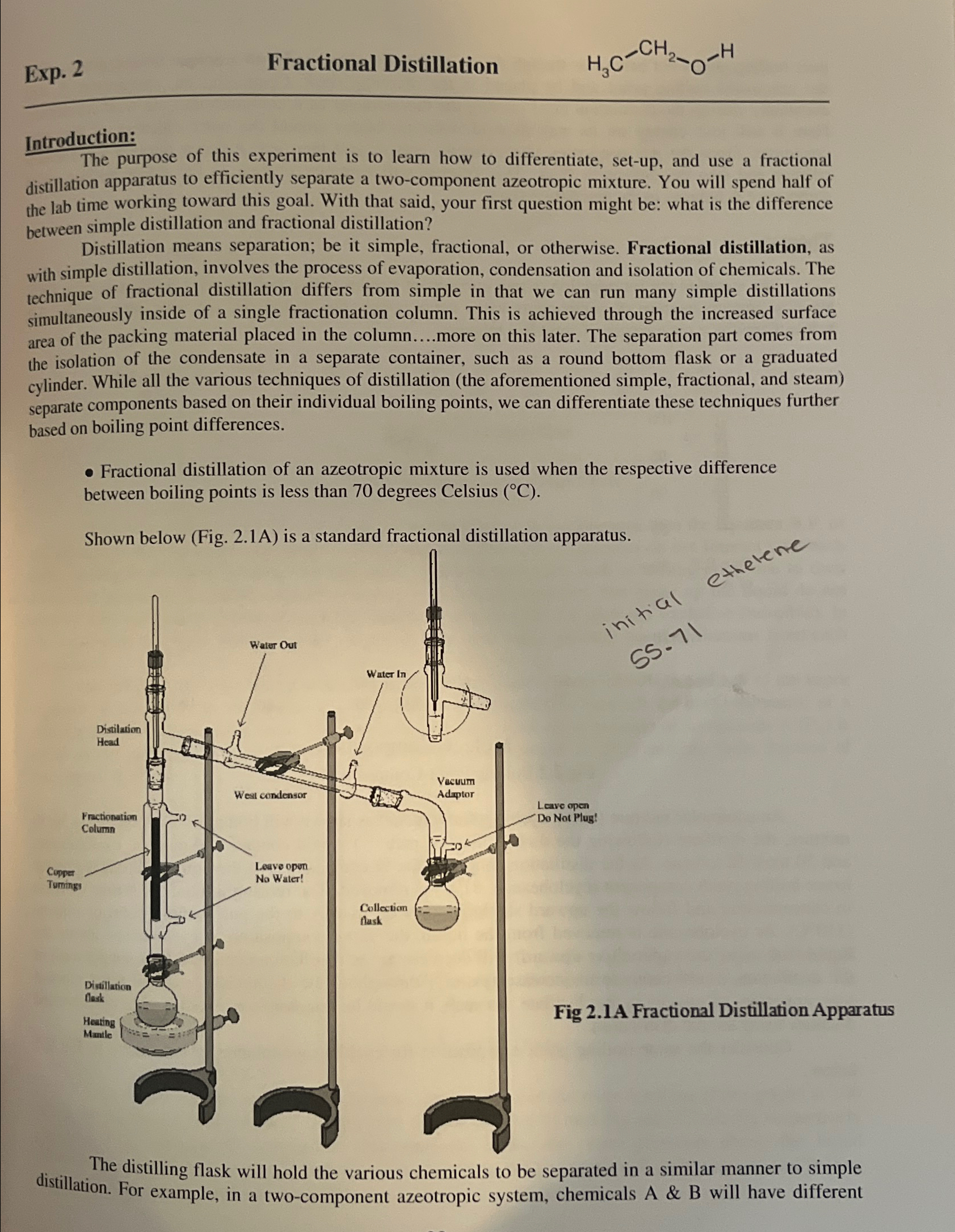
Exp. 2\ Fractional Distillation\ Introduction:\ The purpose of this experiment is to learn how to differentiate, set-up, and use a fractional distillation apparatus to efficiently separate a two-component azeotropic mixture. You will spend half of the lab time working toward this goal. With that said, your first question might be: what is the difference between simple distillation and fractional distillation?\ Distillation means separation; be it simple, fractional, or otherwise. Fractional distillation, as with simple distillation, involves the process of evaporation, condensation and isolation of chemicals. The technique of fractional distillation differs from simple in that we can run many simple distillations simultaneously inside of a single fractionation column. This is achieved through the increased surface area of the packing material placed in the column....more on this later. The separation part comes from the isolation of the condensate in a separate container, such as a round bottom flask or a graduated cylinder. While all the various techniques of distillation (the aforementioned simple, fractional, and steam) separate components based on their individual boiling points, we can differentiate these techniques further based on boiling point differences.\ Fractional distillation of an azeotropic mixture is used when the respective difference between boiling points is less than 70 degrees Celsius
(\\\\deg C).\ Shown below (Fig. 2.1A) is a standard fractional distillation apparatus.\ The distilling flask will hold the various chemicals to be separated in a similar manner to simple distillation. For example, in a two-component azeotropic system, chemicals A & B will have different
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


